Compounds for treating parvovirus infection
A technology of parvoviruses and compounds, applied in the field of compounds, can solve problems such as amantadine treatment or prevention of parvovirus infections that have not been proposed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
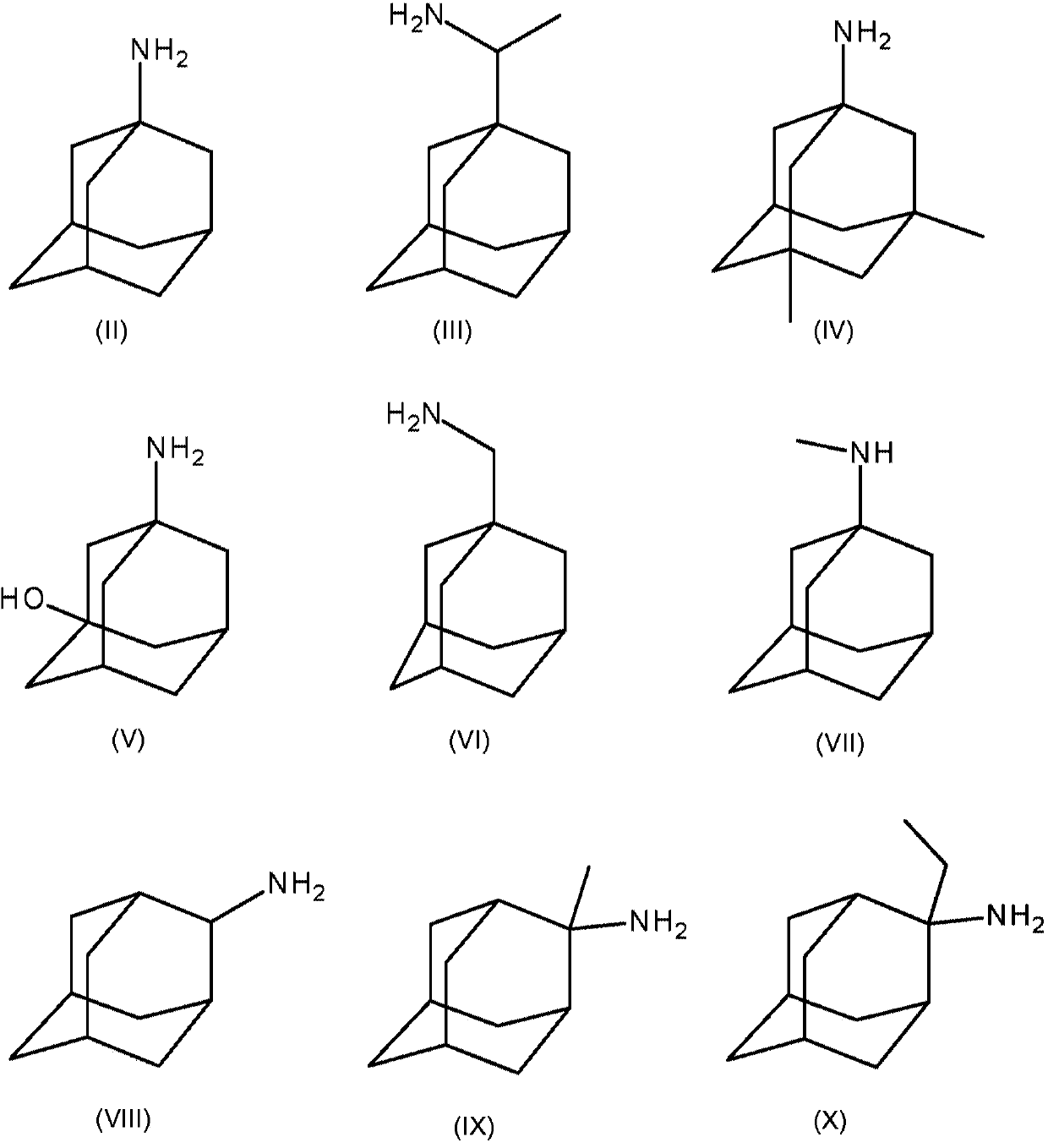
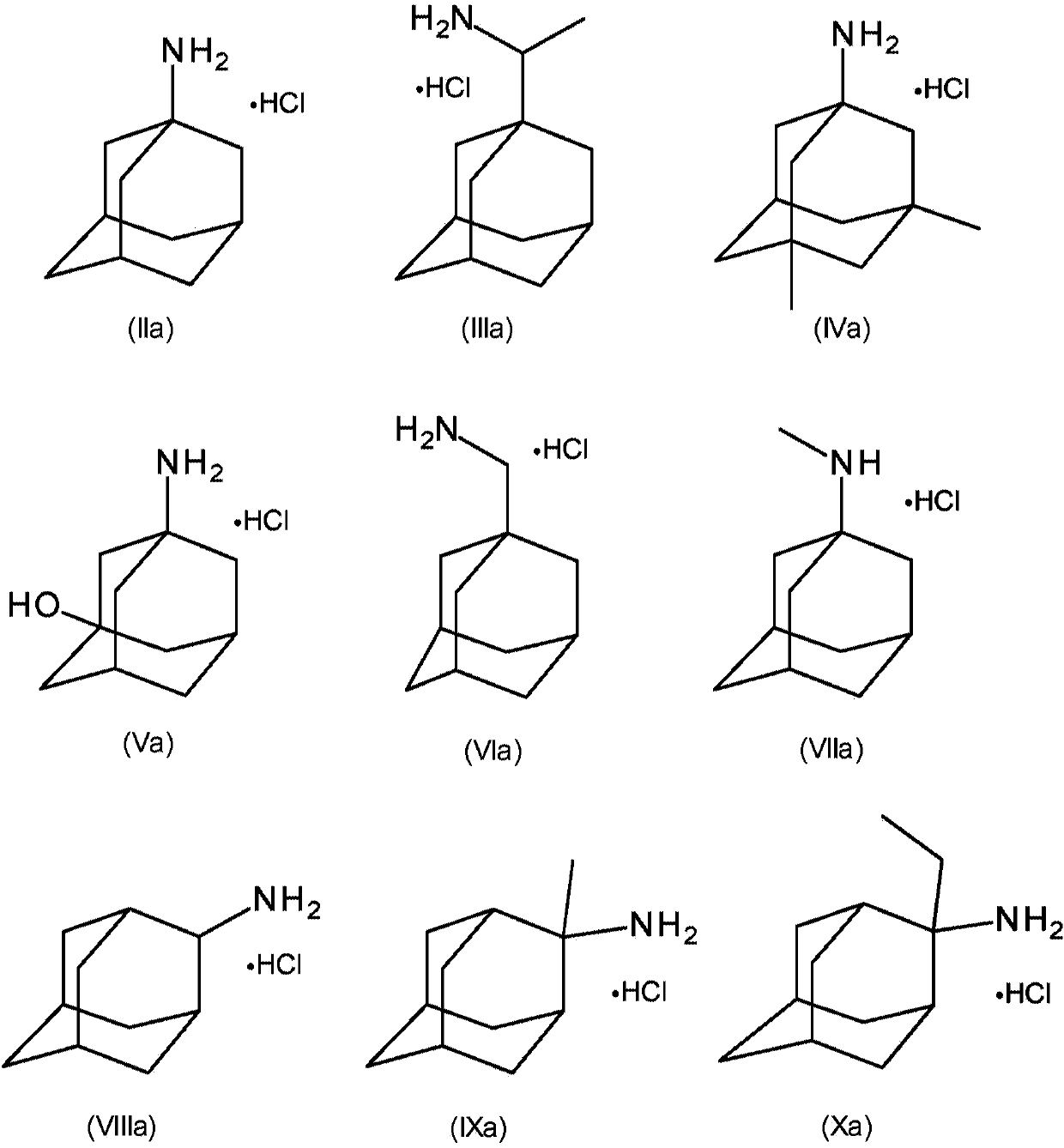
[0119] Example 1: Through amantadine, amantadine hydrochloride (HCl), 2-adamantylamine HCl, 3-amino-1-adamantanol (amantadol), N-methyl-1-adamantyl Amine, 1-adamantylmethylamine, rimantadine, and memantine inhibit feline panleukopenia virus and canine parvovirus 2c replication in cell culture
[0120] Materials and methods
[0121] Add amantadine, amantadine HCl, 2-adamantylamine HCl, 3-amino-1-adamantyl alcohol (amantadol), N-methyl-1-adamantylamine, 1-adamantylamine at a concentration of 10 mM Alkylmethylamines, rimantadine and memantine were dissolved in Dulbecco's phosphate buffered saline (DPBS). Crandell Reese feline kidney (CrFK) cells were grown in Dulbecco's minimal essential medium containing 1% sodium bicarbonate (Life Technologies), 1% L-glutamine (Life Technologies), and 5% fetal calf serum (FCS, Biochrom) (DMEM, Life Technologies). Add serial dilutions of compounds along with 10 TCID in a 96-well plate 50 Feline panleukopenia virus (FPV) or 10TCID 50 Canin...
Embodiment 2
[0126] Example 2: Inhibition of feline panleukopenia virus and canine parvovirus 2c production and viral DNA production in cell culture by amantadine, amantadine hydrochloride (HCl) and memantine.
[0127] Materials and methods
[0128] Amantadine, amantadine hydrochloride (HCl) and memantine were dissolved in Dulbecco's phosphate buffered saline (DPBS) at a concentration of 10 mM. Crandell Reese feline kidney (CrFK) cells were cultured in Dulbecco's minimal essential medium containing 1% sodium bicarbonate (Life Technologies), 1% L-glutamine (Life Technologies), and 5% fetal calf serum (FCS, Biochrom) (DMEM, Life Technologies). 100,000 CrFK cells were seeded in 24-well plates on day 0. On day 1, remove medium and add 10 TCID 50 FPV or 10TCID 50 CPV-2c and incubated for 2 h at 37°C under humidified conditions. After incubation, the medium was removed and the CrFK cells were washed with DPBS. Subsequently, serial dilutions (250, 50 and 10 [mu]M) of the compound in the m...
Embodiment 3
[0138] Example 3: Inhibition of feline panleukopenia virus and canine parvovirus 2c production in cell culture by 2-methyladamantan-2-amine hydrochloride and 2-ethyladamantan-2-amine hydrochloride and viral DNA production.
[0139] 2-Methyladamantan-2-amine HCl and 2-ethyladamantan-2-amine HCl were dissolved in Dulbecco's phosphate buffered saline (DPBS) at a concentration of 10 mM. Its inhibition experiments and preparation were performed as described above for Example 2.
[0140] 2-Methyladamantan-2-amine HCl and 2-Ethyl Glycine effectively inhibited 50% of FPV and CPV-2c virus and DNA production as determined by cytopathic effect (CPE) visual scoring and measured by quantitative PCR The concentration of adamantane-2-amine HCl (EC 50 ) are given in Table 4a and Table 4b, respectively. Both 2-methyladamantan-2-amine HCl and 2-ethyladamantan-2-amine HCl were found to be very effective in reducing viral DNA.
[0141] Table 4a - EC in μM for antiviral activity of compounds a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com