AAV1 virus medicated PCK1 gene expression vector with skeletal muscle specificity and application thereof
A gene and virus technology, applied in the biological field, can solve problems such as unclear metabolism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1 Construction of AAV1-HSA-PCK1 recombinant expression vector
[0059] According to literature reports, the human α-skeletal muscle actin promoter sequence was obtained (Mol Cell Biol. 1987; 7(11):4089-4099. J Biol Chem. 2007; 282(45): 32844-32855.), and the The promoter sequence was mutated to eliminate the XhoI and SalI restriction sites in the sequence. Then the MVM intron sequence was added behind the promoter to obtain the complete promoter sequence required to express the PCK1 gene. This sequence is named HSA.
[0060] According to the GenBank database, the cDNA sequence of the PCK1 gene was obtained. According to the principle of codon degeneracy, the obtained PCK1 gene coding sequence was mutated to eliminate the restriction sites of EcoRI and BglII. Four fully complementary target sequences of miR-122 (highly expressed in the liver) were then introduced after the stop codon of the PCK1 gene. The sequence was named PCK1-4×miR-122T.
[0061] The two s...
Embodiment 2A
[0063] Packaging and assay of embodiment 2AAV1-HSA and AAV1-HSA-PCK1 recombinant virus
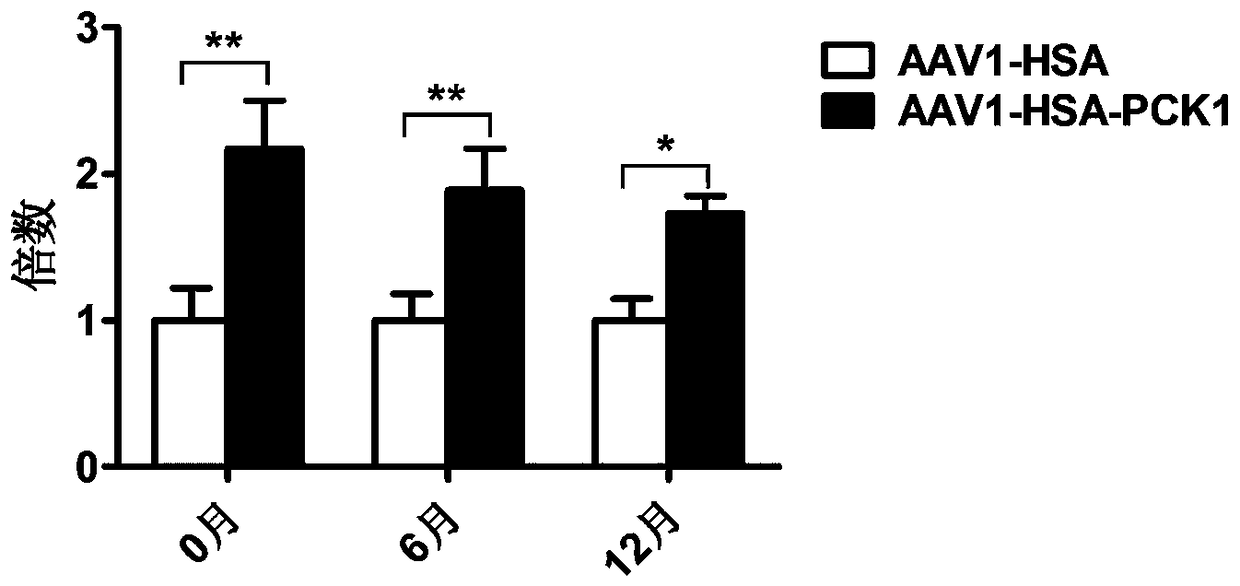
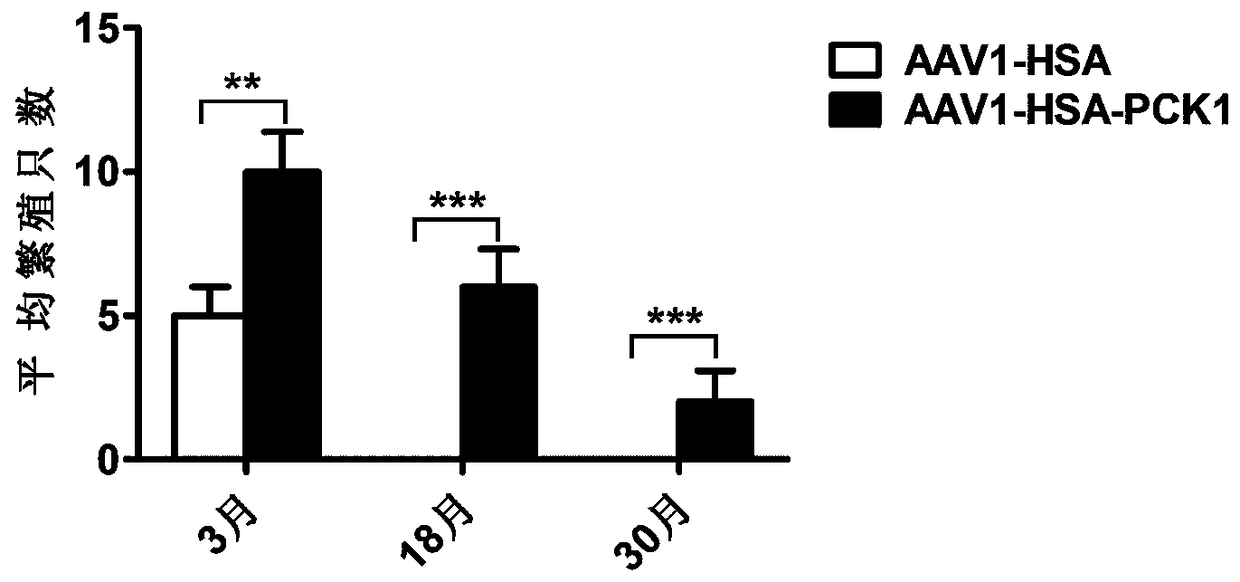
[0064] Referring to literature (Xiao X, et al. Production of High-Titer Recombinant Adeno-Associated Virus Vectors in the Absence of Helper Adenovirus. J Virol. 1998; 72(3):2224-2232.), the application of three plasmid packaging system packaging and Purification of recombinant AAV virus. Briefly, the AAV vector plasmid (pAAV2neo-HSA or pAAV2neo-HSA-PCK1-4×miR-122T), the helper plasmid (pHelper) and the AAV1 Rep and Cap protein expression plasmid pAAV-R2C1 were used in a molar ratio of 1:1:1 After mixing, HEK293 cells were transfected by the calcium phosphate method. After 48 hours of transfection, the cells and culture supernatant were harvested, and the recombinant AAV virus was isolated and purified by cesium chloride density gradient centrifugation. Two recombinant viruses, AAV1-HSA (packaged from pAAV2neo-HSA plasmid) and AAV1-HSA-PCK1 (packaged from pAAV2neo-HAS-PCK1-4×miR-122T plasm...
Embodiment 3
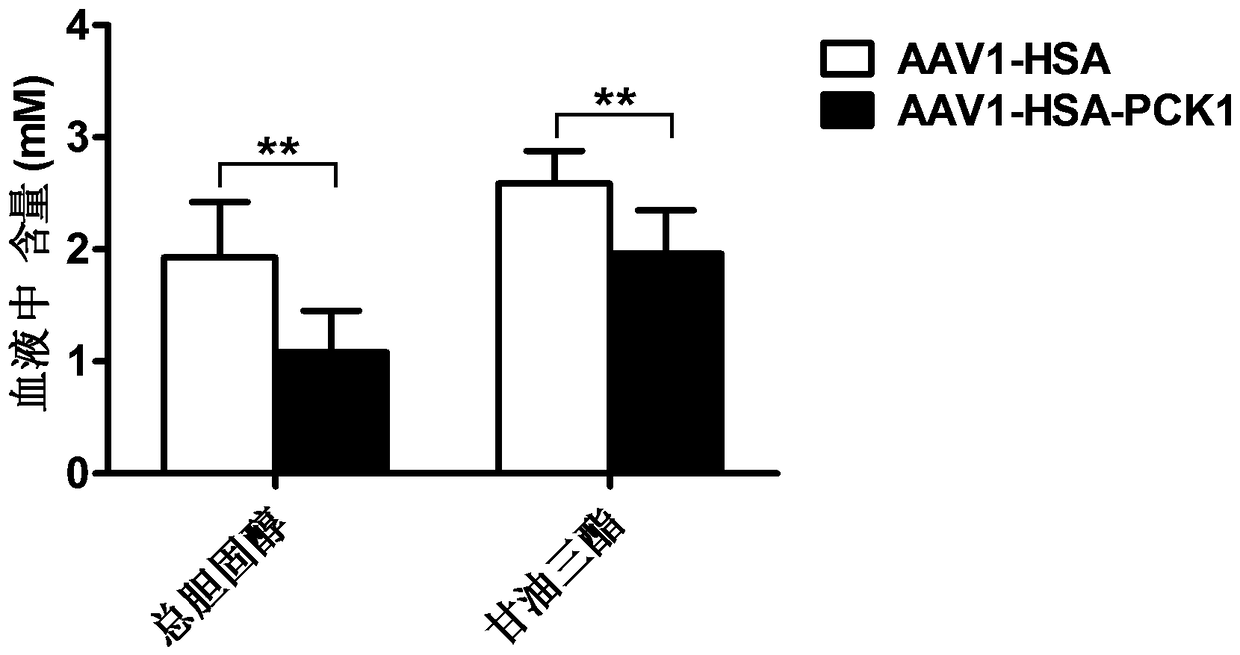
[0070] Example 3 Establishment of hyperlipidemia mouse animal model
[0071] Twelve mice of C57BL / 6J strain, half male and half male, weighing 18-22g, were raised under the conditions of relative humidity 60±10% and temperature 22±1ºC. After adaptively feeding the mice with ordinary feed for 3 days, they switched to high-fat feed (33.5% corn meal, 5.0% fish meal, 2.0% cholesterol, 4.0% milk powder, 10.0% palm oil, 16.7% soybean and other nutrients), The hyperlipidemia model was successfully established by continuous feeding for 16 weeks. After that, the mice were randomly divided into control group and experimental group, with half male and half male in each group. The dose of multi-point injection into the muscle of the control group and the experimental group was 1×10 11 vg / AAV1-HSA and AAV1-HSA-PCK1 virus, continue to feed for one month, remove the eyeball to take blood (12 hours before blood collection, fasting without water), centrifuge at 3000rpm for 10min to take seru...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com