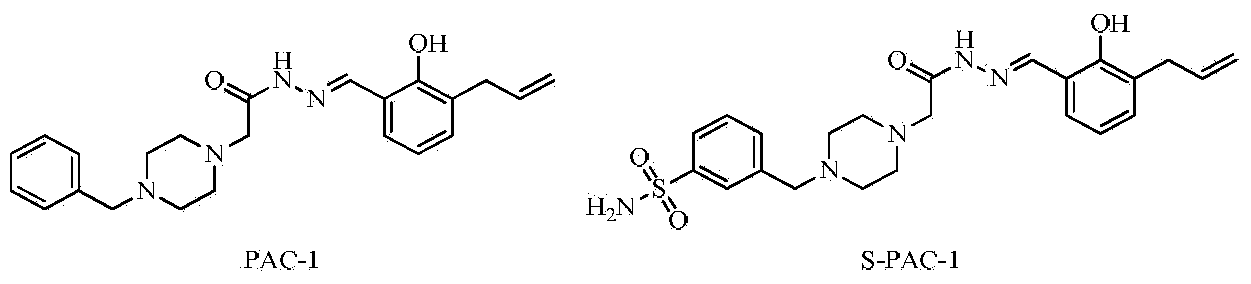
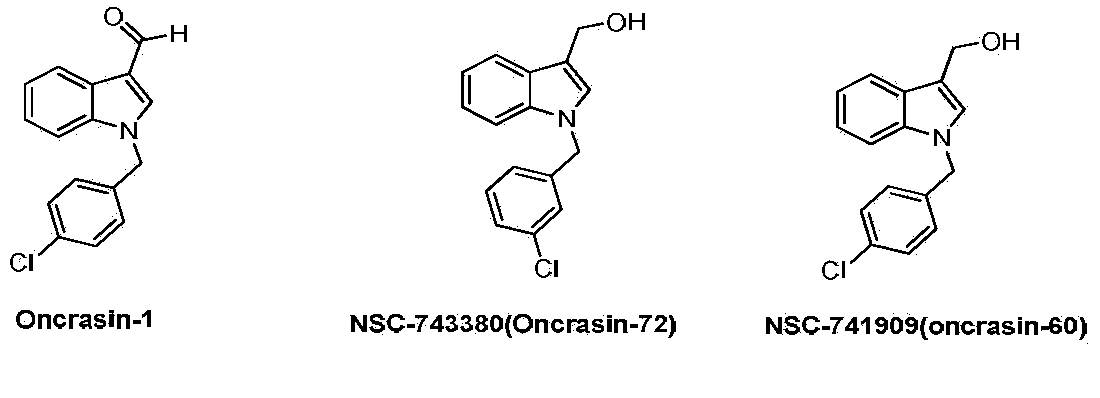
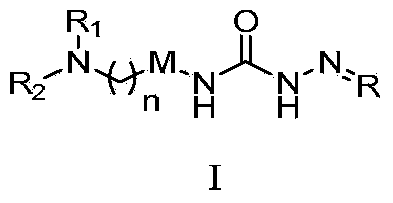
Semicarbazone derivatives and application thereof
A technology of alkyl and compound, applied in the field of medicine, can solve problems such as toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0317] Example 1: (E)-N 1 -[2-(6-(4-Methylpiperidinyl)methylbenzo[d]thiazolyl)]-N 4 Preparation of -(2,4-dimethoxybenzaldehyde)semicarbazone
[0318] The preparation of step A N-(4-nitrobenzyl) diethylamine (1)
[0319] Add p-nitrobenzyl bromide (10.8g, 0.05mol) to 50mL of acetonitrile, add 10 times the amount of diethylamine, react at room temperature for 3h, add water to the reaction system, extract with dichloromethane three times, and dry the dichloromethane layer , evaporated to dryness to give 9.4g yellow liquid, yield: 90%, MS [MH + ] (m / z): 209.3.
[0320] Preparation of step B 4-(diethylaminomethyl)aniline (2)
[0321] Add intermediate 1 (10.4g, 0.05mol) to 100mL ethanol, raise the temperature to 70°C, add ferric chloride (2.8g, 0.001mol) and activated carbon (0.18g, 0.015mol), keep at 70°C, drop Add hydrazine hydrate (25g, 0.5mol), drop it, reflux for 5h, suction filter while it is hot, concentrate the ethanol solution, add water, extract three times with dichlo...
Embodiment 2
[0332] Example 2(E)-N 1 -[2-(6-(Diethylamino)methylbenzo[d]thiazolyl)]-N 4 -(4-Methoxybenzaldehyde)semicarbazone
[0333] ESI-MS [M+H] (m / z): 412.2;
Embodiment 3
[0334] Example 3(E)-N 1 -[2-(6-(Diethylamino)methylbenzo[d]thiazolyl)]-N 4 -(3-bromo-4-hydroxybenzaldehyde)semicarbazone
[0335] ESI-MS [M+H] (m / z): 476.1;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com