A kind of luminol chemiluminescence system and the determination method of luminol, thiourea dioxide, cobalt ion concentration
A technology of thiourea dioxide and luminol chemistry, which is applied in the field of chemical analysis, can solve the problems of unstable hydrogen peroxide, slow chemiluminescence reaction, and low luminescence intensity, and achieves improved luminescence intensity, good thermal stability, and high luminescence intensity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
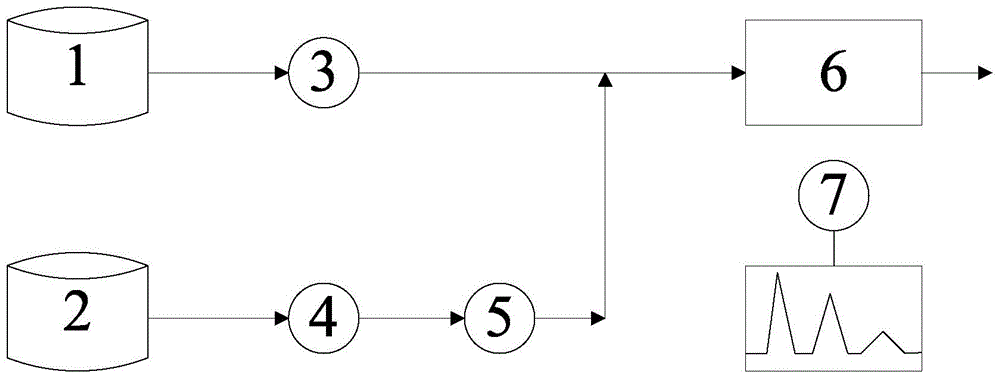
[0070] use figure 1 The system shown measures the concentration of luminol
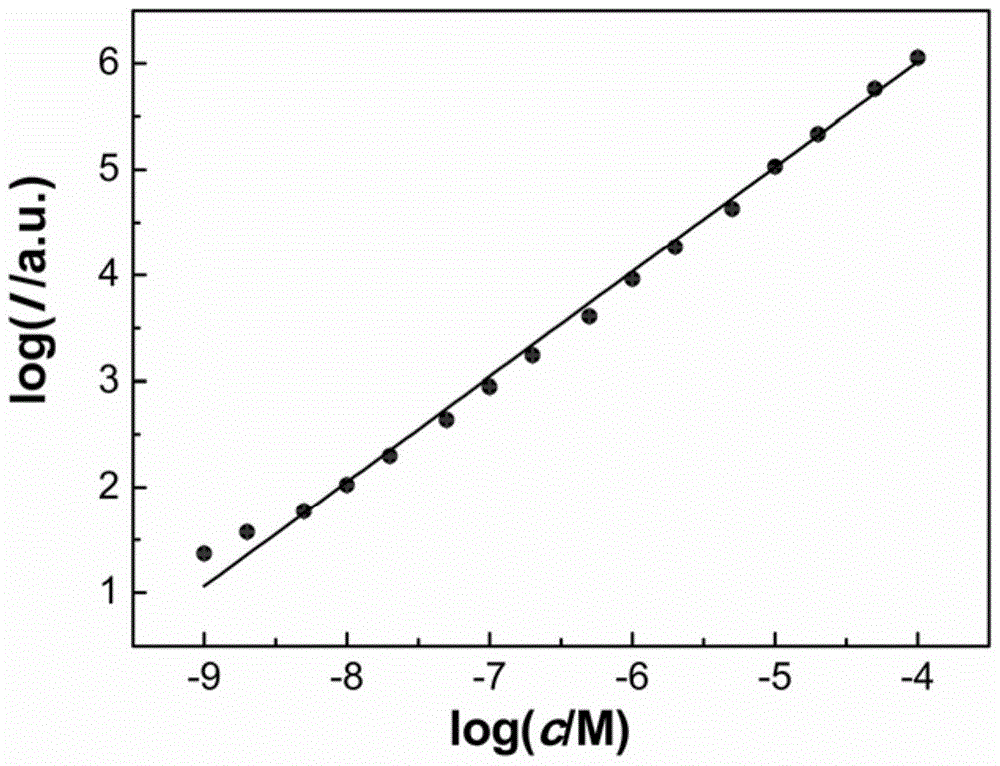
[0071] Prepare 40mmol / L thiourea dioxide solution, sodium carbonate-sodium hydroxide buffer solution with a pH value of 11.9 and a concentration of 1×10 -9 mol / L, 2×10 -9 mol / L, 5×10 -9 mol / L, 1×10 -8 mol / L, 2×10 -8 mol / L, 5×10 -8 mol / L, 1×10 -7 mol / L, 2×10 -7 mol / L, 5×10 -7 mol / L, 1×10 -6 mol / L, 2×10 -6 mol / L, 5×10 -6 mol / L, 1×10 -5 mol / L, 2×10 -5 mol / L, 5×10 -5 mol / L, 1×10 -4 mol / L luminol solution.
[0072] Add 50 mL of 40 mmol / L thiourea dioxide solution to the first container, add 50 mL of sodium carbonate-sodium hydroxide buffer solution with a pH value of 11.9 to the second container, start the first and second pumping units, and set the flow rate to 1.25 mL / min. In turn, the concentration was 1×10 -9 mol / L, 2×10 -9 mol / L, 5×10 -9 mol / L, 1×10 -8 mol / L, 2×10 -8 mol / L, 5×10 -8 mol / L, 1×10 -7 mol / L, 2×10 -7 mol / L, 5×10 -7 mol / L, 1×10 -6 mol / L, 2×10 -6 mol / L, 5×10 -6 mo...
Embodiment 2
[0076] use figure 1 The system shown measures the concentration of thiourea dioxide
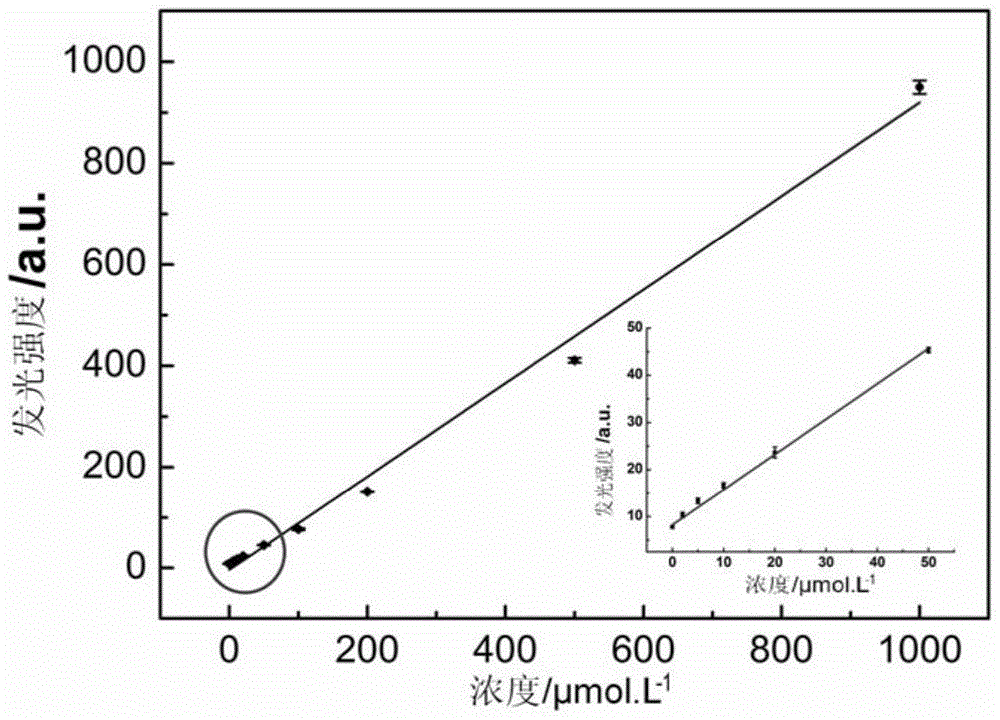
[0077] Prepare an alkaline solution of luminol (10 μmol / L, pH 11.9) and a concentration of 0, 2×10 -6 mol / L, 5×10 -6 mol / L, 1×10 -5 mol / L, 2×10 -5 mol / L, 5×10 -5 mol / L, 1×10 -4 mol / L, 2×10 -4 mol / L, 5×10 -4 mol / L, 1×10 -3 mol / L solution of thiourea dioxide.
[0078] Add 50 mL of an alkaline solution of luminol to the first container, add 50 mL of water to the second container, start the first and second pumping units, and set the flow rate to 1.25 mL / min. Set the concentration to 0, 2×10 in turn -6 mol / L, 5×10 -6 mol / L, 1×10 -5 mol / L, 2×10 -5 mol / L, 5×10 -5 mol / L, 1×10 -4 mol / L, 2×10 -4 mol / L, 5×10 -4 mol / L, 1×10 -3 50 μL of mol / L thiourea dioxide was injected into the injection valve, and the chemiluminescence detector recorded the luminous intensity under different thiourea dioxide concentrations (the voltage of the photomultiplier tube PMT was 1000V).
[0079] Take the c...
Embodiment 3
[0082] use figure 1 The system shown measures cobalt ion concentration
[0083] Preparation of alkaline solution of luminol (10 μmol / L, pH11.9), 0.1mol / L thiourea dioxide solution and concentrations of 0.5nmol / L, 5nmol / L, 10nmol / L, 50nmol / L, 100nmol / L, 200nmol / L, 400nmol / L, 600nmol / L, 800nmol / L, 1000nmol / L cobalt chloride solution, 10μL of the thiourea dioxide solution and 1mL of water were mixed to obtain a blank control sample, and 10μL of the Thiourea dioxide solution and 1 mL of the cobalt chloride solution were mixed to obtain a series of mixed solutions.
[0084] Add 50 mL of an alkaline solution of luminol to the first container, add 50 mL of water to the second container, start the first and second pumping units, and set the flow rate to 1.25 mL / min. The blank control sample and the mixed solution were sequentially injected into the injection valve, each injection volume was 50 μL. The chemiluminescence detector records the luminous intensity under different cobalt ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com