Preparation device and preparation method of universal virus inactivation blood plasma
A virus inactivation and preparation device technology, which is applied in the direction of medical raw materials derived from mammals, can solve the problems of cumbersome operation, low preparation efficiency, and failure to meet application requirements, so as to facilitate storage and transportation, improve preparation efficiency, and improve Effect of Infusion Efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] The content of the invention of the present invention will be further described below in conjunction with the accompanying drawings and embodiments.
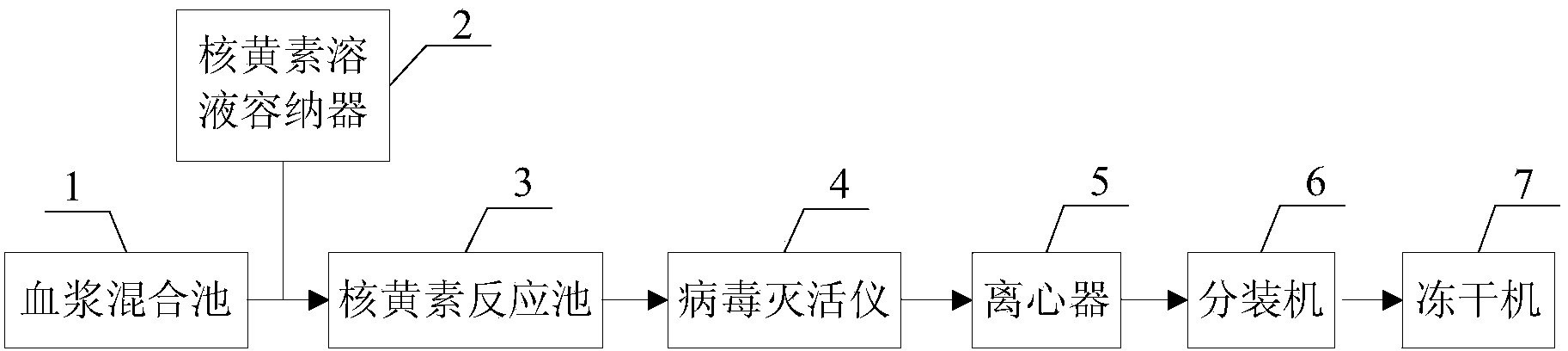
[0031] Such as figure 1 As shown, the general-purpose virus inactivated plasma preparation device provided in this embodiment includes a plasma mixing pool 1 , a riboflavin solution container 2 , a riboflavin reaction pool 3 , a virus inactivation instrument 4 and a centrifuge 5 . Both the plasma mixing pool 1 and the riboflavin solution container 2 are connected to the riboflavin reaction pool 3 through pipelines, and the riboflavin reaction pool 3 is connected to the virus inactivation instrument 4 and the centrifuge 5 through pipelines in turn. Preferably, a first agitator (not shown in the figure) is installed at the bottom of the plasma mixing pool 1, and a second agitator (not shown in the figure) is installed at the bottom of the riboflavin reaction pool 3.
[0032] In a preferred implementation of this embodiment...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com