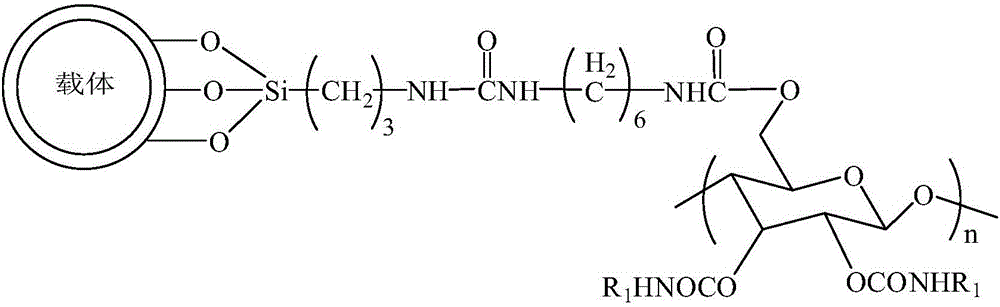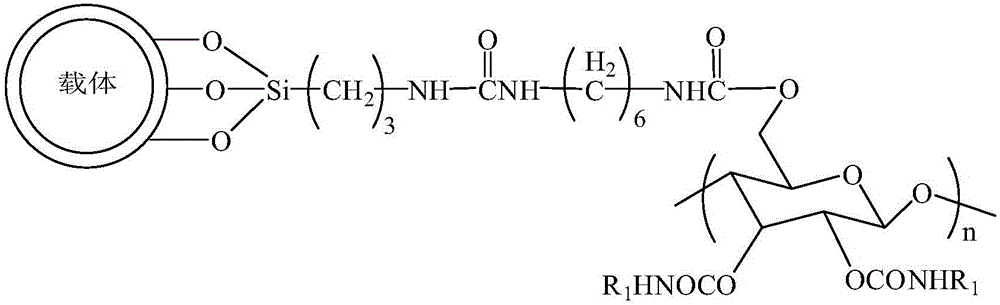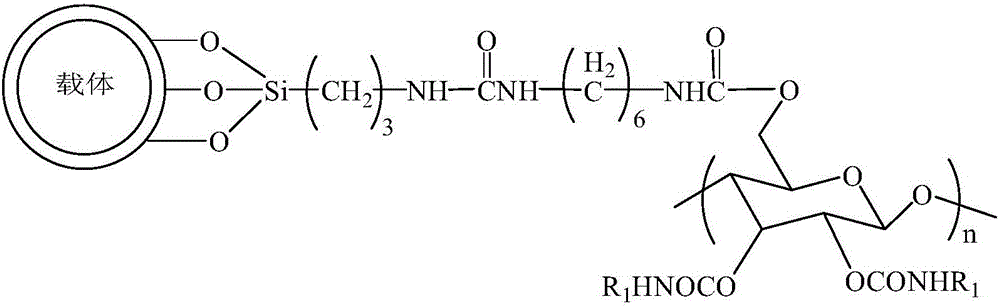A chiral stationary phase bonded with amylose derivatives and its preparation method
A technology of chiral stationary phase and amylose, applied in chemical instruments and methods, and other chemical processes, can solve the problems of insufficient chiral selectivity and instability, and achieve good selectivity, stability, and excellent The effect of chiral selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation of the chiral stationary phase bonded with amylose derivatives, the specific process is as follows:
[0035] Step 1: Take 10g of styrene-divinylbenzene polymer-coated silica gel and add 300mL of concentrated hydrochloric acid and water with a volume ratio of 1:1. Under electromagnetic stirring, heat and reflux in an oil bath at 90°C for 4h, cool, stand still, and pour out Acid solution, washed several times with water until it becomes neutral; after drying, add 70mL toluene and 1g aminopropyltriethoxysilane, react at 100°C for 24h, add 15mL trimethylchlorosilane for reaction, and use After washing and drying with toluene, diethyl ether and methanol, a styrene-divinylbenzene polymer-coated silica gel containing aminopropyl groups on the surface is prepared. The number of aminopropyl groups on the surface of styrene-divinylbenzene polymer coated silica gel is controlled by the amount of trimethylchlorosilane to 0.8 μmol / m 2 .
[0036] Step 2: Suspend 1g ...
Embodiment 2
[0045]The preparation of the chiral stationary phase bonded with amylose derivatives, the specific process is as follows:
[0046] Step 1: Add 10g of styrene-divinylbenzene polymer-coated silica gel to 300mL of concentrated hydrochloric acid and water with a volume ratio of 1:1, under electromagnetic stirring, heat and reflux in an oil bath at 90°C for 4h, cool, stand still, and pour out the acid solution, washed several times with water until neutral. After drying, add 70mL of toluene and 25g of aminopropyltriethoxysilane, and react at 105°C for 24h. Add 15mL of trimethylchlorosilane for reaction, the reaction solution is cooled, washed and dried with toluene, diethyl ether and methanol successively, and then a styrene-divinylbenzene polymer-coated silica gel containing aminopropyl groups on the surface is obtained. The number of aminopropyl groups on the surface of silica gel coated with styrene-divinylbenzene polymer is controlled to 0.6 μmol / m by adding the amount of trim...
Embodiment 3
[0051] The preparation of the chiral stationary phase bonded with amylose derivatives, the specific process is as follows:
[0052] Step 1: Add 10g of styrene-divinylbenzene polymer-coated silica gel to 300mL of concentrated hydrochloric acid and water with a volume ratio of 1:1, under electromagnetic stirring, heat and reflux in an oil bath at 90°C for 4h, cool, stand still, and pour out the acid solution, washed several times with water until neutral. After drying, add 70mL of toluene and 0.5g of aminopropyltriethoxysilane, and react at 110°C for 24h. Add 15mL of trimethylchlorosilane for reaction, the reaction solution is cooled, washed and dried with toluene, diethyl ether and methanol successively, and then a styrene-divinylbenzene polymer-coated silica gel containing aminopropyl groups on the surface is obtained. Control the number of aminopropyl groups on the surface of styrene-divinylbenzene polymer coated silica gel by adding the amount of trimethylchlorosilane to 0....
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com