Method for preparing 2, 5-furan diformic acid by water phase catalysis of 5-hydroxymethylfurfural
A technology of hydroxymethylfurfural and furandicarboxylic acid, which is applied in the field of aqueous phase catalytic oxidation of 5-hydroxymethylfurfural, can solve the problems of equipment corrosion, production cost increase, environmental pollution, etc., and achieve easy separation and high yield , the effect of good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
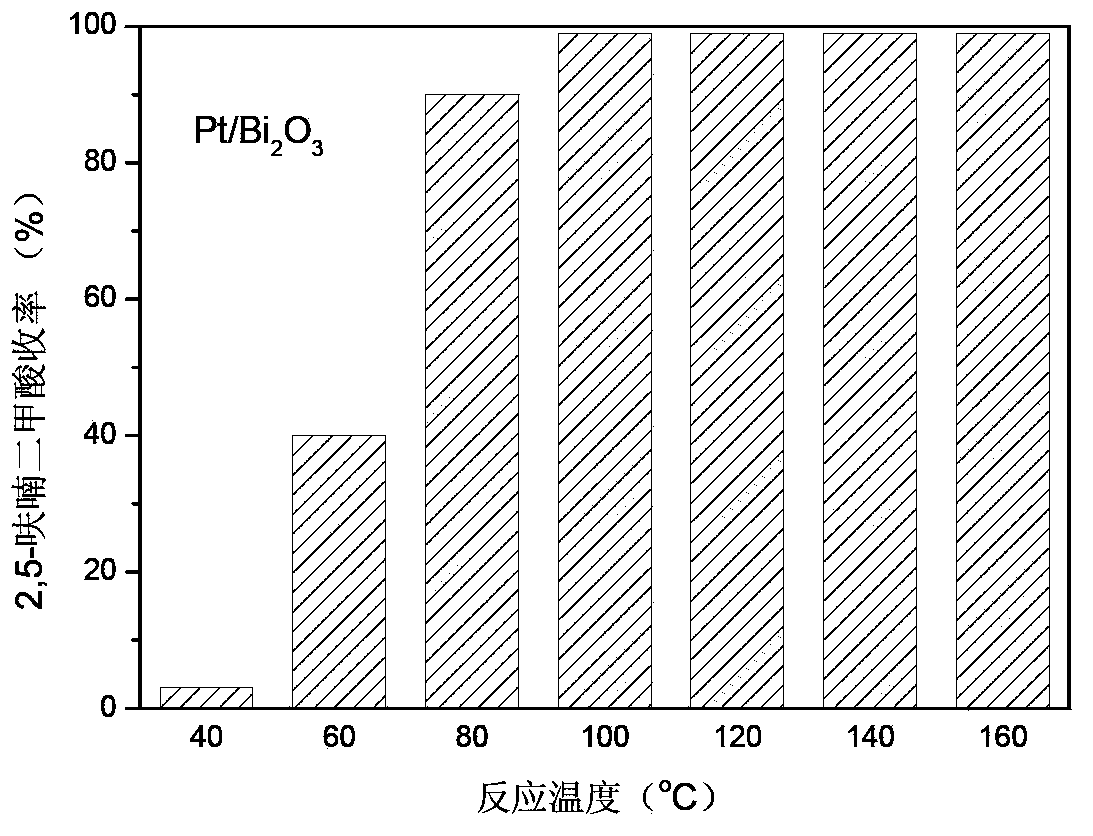
[0023] Example 1-14: Pt / Bi at different temperatures 2 O 3 Or Pd / Bi 2 O 3 Catalytic oxidation of 5-hydroxymethyl furfural to 2,5-furandicarboxylic acid has the following results figure 1 (Pt / Bi 2 O 3 )with figure 2 (Pd / Bi 2 O 3 ) Shown. The specific experimental process is described as follows:
[0024] 0.064g 5-hydroxymethyl furfural, 0.2g Pt / Bi 2 O 3 Or Pd / Bi 2 O 3 (The active component content is 0.2 wt%), 2 mL of deionized water is added to a 10 mL reactor, filled with oxygen to 0.1 MPa, and kept at different temperatures for 5 hours under constant stirring. If the partial pressure of oxygen drops, add oxygen to maintain the pressure of oxygen at about 0.1 MPa. After the reaction, it was cooled to room temperature. Product analysis adopts high performance liquid chromatography. The reaction solution was diluted with 80 mL of water, and then the catalyst was filtered out. After the diluted reaction solution was adjusted to 100 mL, a sample was taken and analyzed by high pe...
Example Embodiment
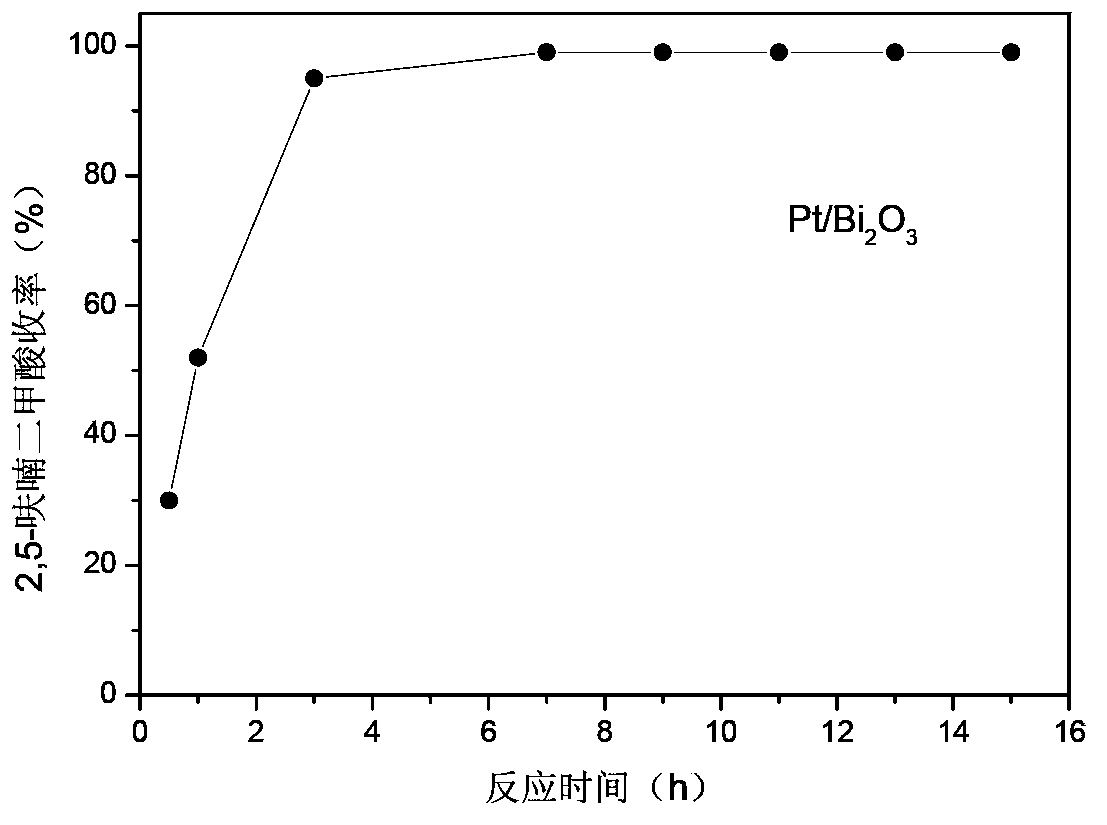
[0026] Example 15-30: Different reaction time, Pt / Bi 2 O 3 Or Pd / Bi 2 O 3 Catalytic oxidation of 5-hydroxymethyl furfural to 2,5-furandicarboxylic acid has the following results image 3 (Pt / Bi 2 O 3 )with Figure 4 (Pd / Bi 2 O 3 ) Shown. The specific experimental process is described as follows:
[0027] 0.064g 5-hydroxymethyl furfural, 0.2g Pt / Bi 2 O 3 Or Pd / Bi 2 O 3 (The active component content is 0.2wt%), 2mL of deionized water is added to a 10mL reactor, filled with oxygen to 0.1MPa, heated to 100°C under constant stirring, and kept for different reaction times. If the partial pressure of oxygen drops, add oxygen to maintain the pressure of oxygen at about 0.1 MPa. After the reaction, it was cooled to room temperature. Product analysis adopts high performance liquid chromatography. The reaction solution was diluted with 80 mL of water, and then the catalyst was filtered out. After the diluted reaction solution was adjusted to 100 mL, a sample was taken and analyzed by hig...
Example Embodiment
[0029] Examples 31-48: Different catalyst amounts, Pt / Bi 2 O 3 Or Pd / Bi 2 O 3 Catalytic oxidation of 5-hydroxymethyl furfural to 2,5-furandicarboxylic acid has the following results Figure 5 (Pt / Bi 2 O 3 )with Image 6 (Pd / Bi 2 O 3 ) Shown.
[0030] The specific experimental process is described as follows:
[0031] 0.064g 5-hydroxymethyl furfural and 2mL deionized water were added to the 10mL reactor, and then respectively take 0.0064g, 0.0256g, 0.0512g, 0.0640g, 0., 1920g, 0.3200g, 0.4480g, 0.5760g, 0.6400g Pt / Bi 2 O 3 Or Pd / Bi 2 O 3 (The active component content is 0.2wt%) is added to it, filled with oxygen to 0.1MPa, heated to 100°C under constant stirring, and kept for 5h. If the partial pressure of oxygen drops, add oxygen. After the reaction, it was cooled to room temperature. Product analysis adopts high performance liquid chromatography. The reaction solution was diluted with 80 mL of water, and then the catalyst was filtered out. After the diluted reaction solution w...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap