Electrophotographic photoreceptor and image forming device
An electrophotographic and photoreceptor technology, which is applied in the electrical recording process using charge patterns, the equipment and optics of the electrical recording process applying charge patterns, etc., can solve the problems such as difficulty in exerting photosensitive characteristics, and achieve the effect of excellent photosensitive characteristics.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
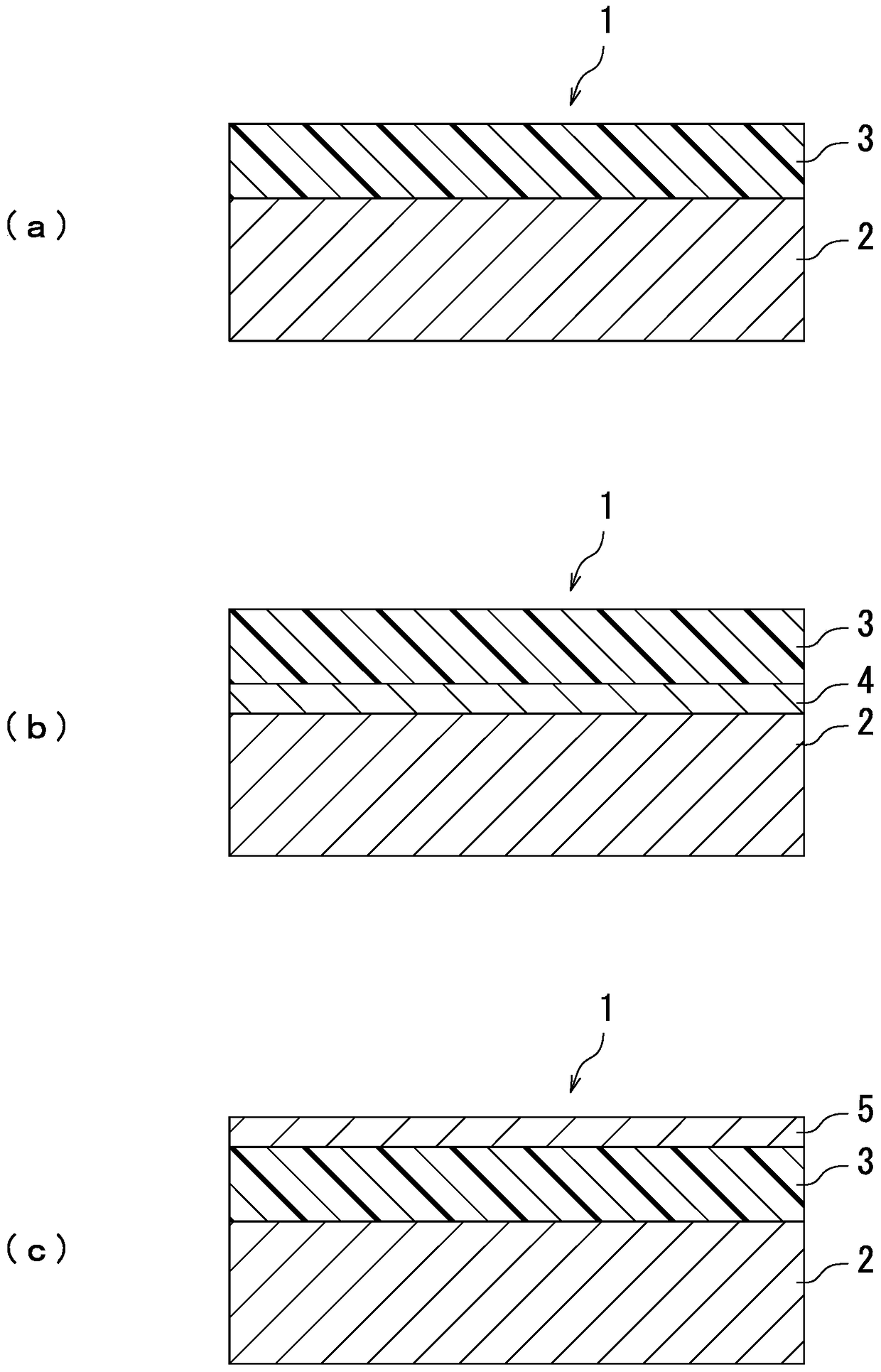
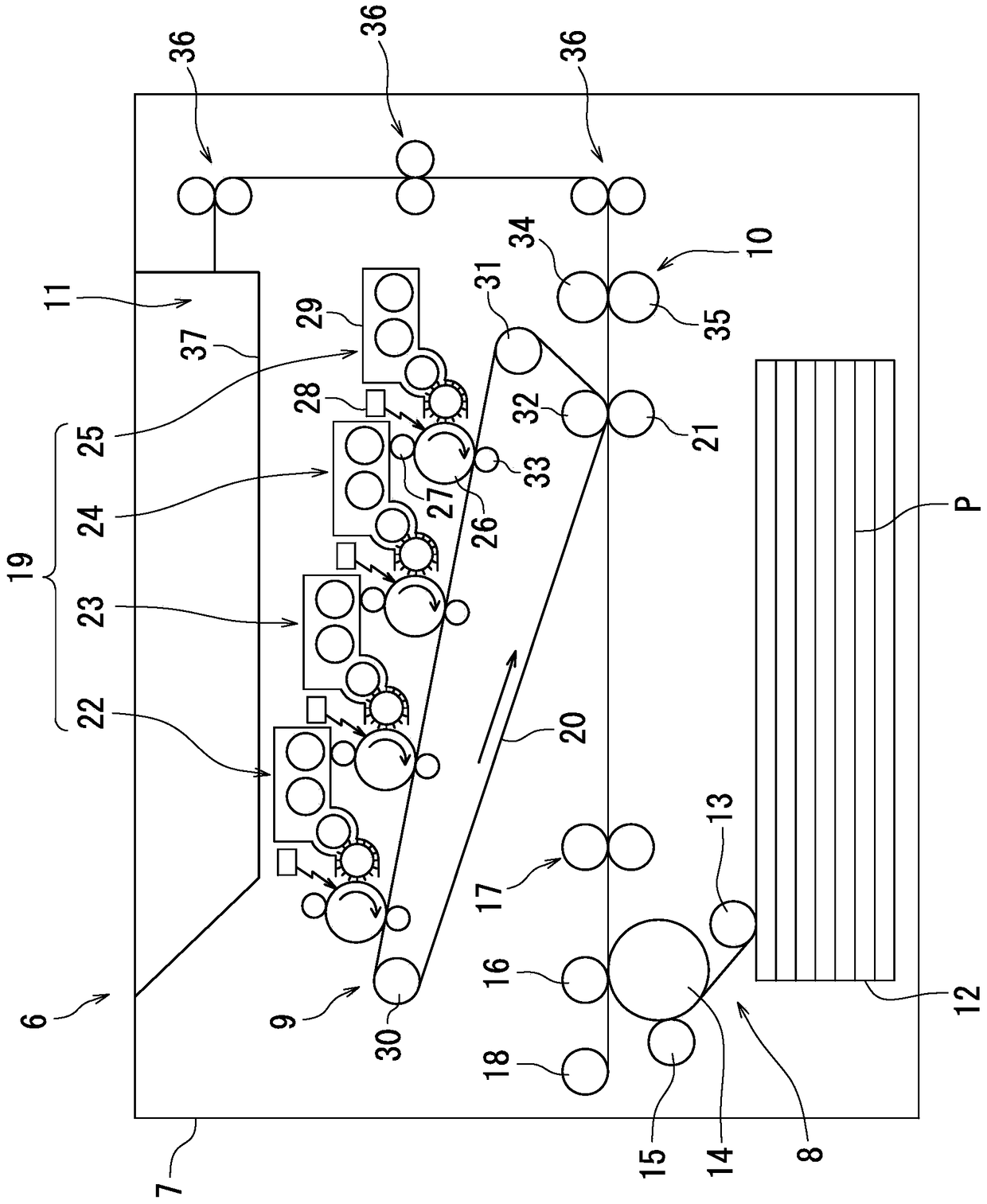
Image
Examples
Embodiment
[0105] Hereinafter, the present invention will be further specifically described through examples. In addition, this invention is not limited at all by an Example.
[0106] [Synthesis of terphenylquinone derivatives]
Synthetic example 1
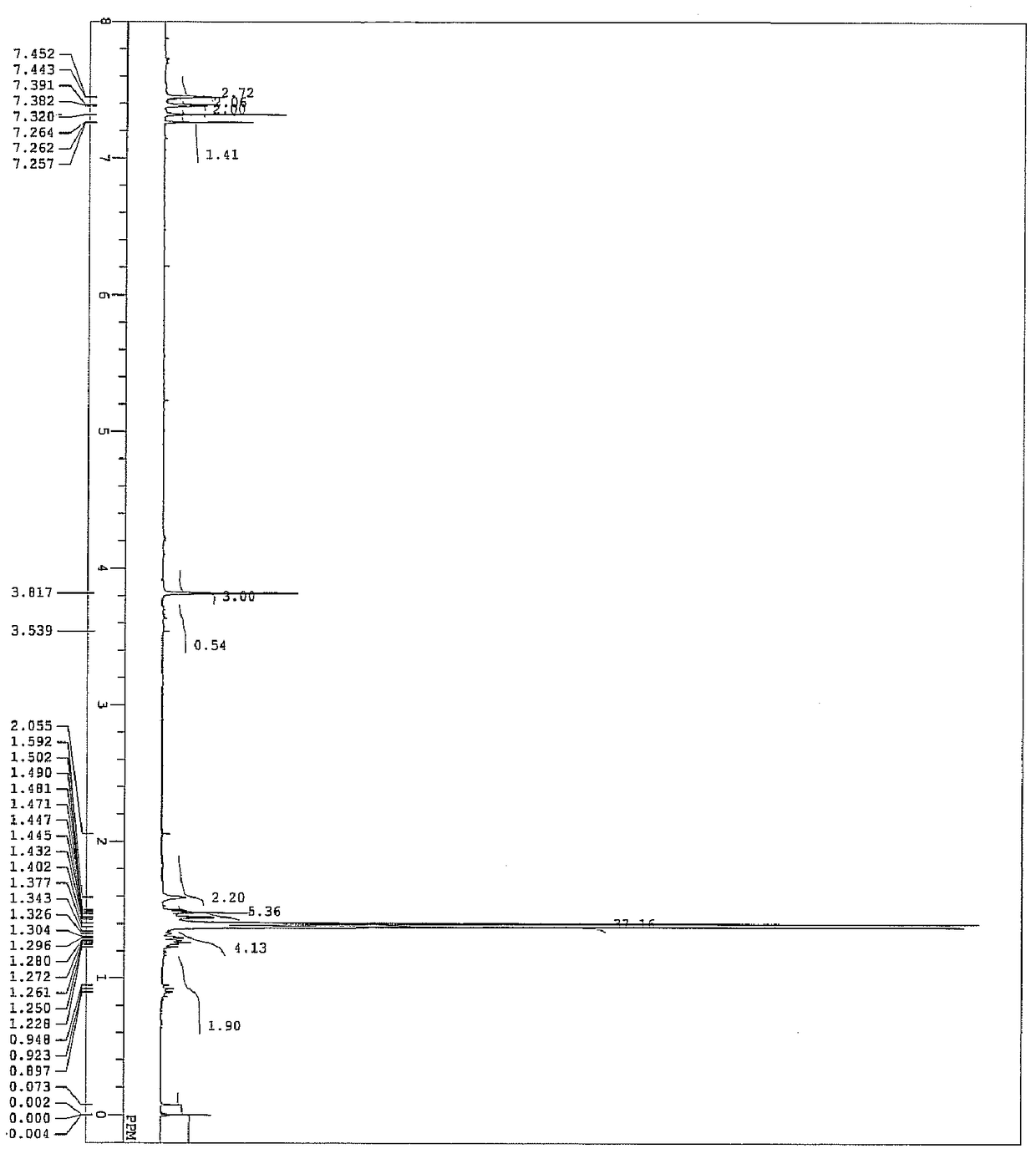
[0108] According to the following reaction formulas (V) to (VIII), terphenylquinone derivatives represented by the following general formula (1-1) are synthesized.
[0109] [chemical formula 6]
[0110]
[0111] [chemical formula 7]
[0112] Reaction formula (V)
[0113]
[0114] [chemical formula 8]
[0115] Reaction formula (VI)
[0116]
[0117] [chemical formula 9]
[0118] Reaction formula (VII)
[0119]
[0120] [chemical formula 10]
[0121] Reaction formula (VIII)
[0122]
[0123] In the reaction formula (V), 4-iodo-2,6-di-tert-butylphenol (A-9) was cooled to 20°C in a tetrahydrofuran (THF) solution of 6.7 g (20 mmol), and 1.6 M concentration n-BuLi n-hexane solution 13mL (21mmol). By further adding trimethylchlorosilane (TMSCl), 4-iodo-2,6-di-tert-butylphenol (yield 3.6 g, yield 45%) with hydroxyl protected by trimethylsilyl (TMS) was obtained (A -10).
[0124] In reaction formula (VI), the THF solution of compound (A-11) (340mg, 4.2mmol) was...
Synthetic example 2
[0128] In Reaction Formula (V), except changing Compound (A-9) to Compound (A-17), and replacing Compound (A-10) to synthesize 1.34g (4.2mmol) of Compound (A-18) to use In addition, by performing the same operation as in Synthesis Example 1, a terphenylquinone derivative represented by the following general formula (1-2) was obtained (0.48 g, 15% yield, purple crystal).
[0129] [chemical formula 11]
[0130]
[0131] [chemical formula 12]
[0132]
[0133] [chemical formula 13]
[0134]
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com