Compositions and methods of obtaining and using endoderm and hepatocyte cells
A technology of endoderm cells and hepatocytes, applied in biochemical equipment and methods, hepatocytes, embryonic cells, etc., can solve problems such as low efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0393] Example 1: Methods and materials for endoderm differentiation
[0394] endoderm markers
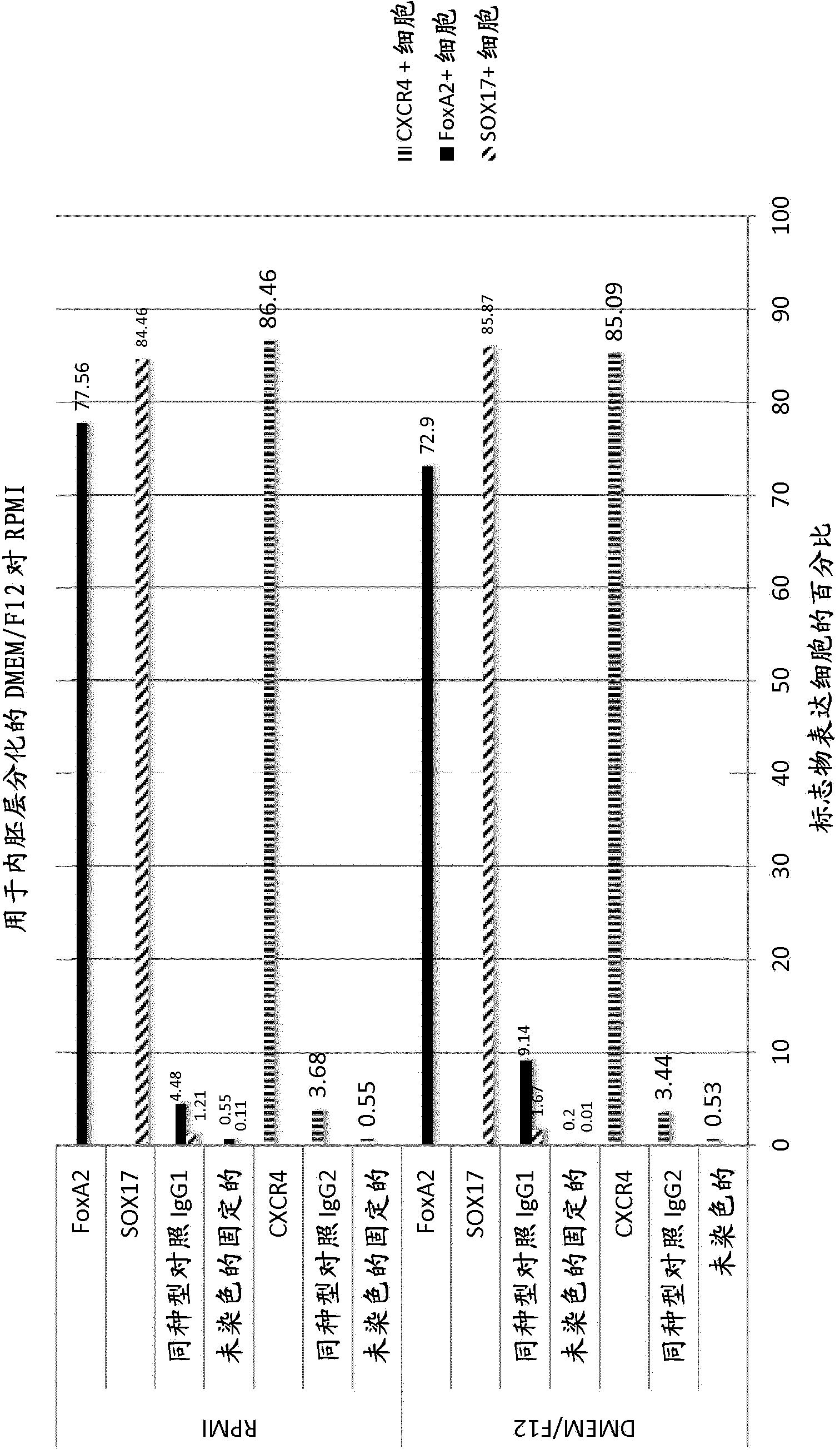
[0395] A variety of cell type-specific markers were used to monitor differentiation of stem cells into endoderm cells in flow cytometry, fluorescence imaging, and immunoassay experiments as described below. To detect endoderm transformation, hESC-derived cell samples were stained for the expression of SOX17, FoxA2 and CXCR4 proteins. SOX17, FoxA2 and CXCR4 are proteins expressed by endoderm cells but not stem cells. To detect stem cells, cell samples were stained for expression of OCT4, a protein expressed by stem cells but not endoderm cells.
[0396] Endoderm Differentiation Protocol Using hESCs and Matrigel
[0397] in TesR TM 2 Medium (STEMCELL TM 40,000 cells / cm on qualified Matrigel (BD, #354277) in Technologies #05860) 2 Density maintenance of undifferentiated human embryonic stem cells (hES). Cultures were manually passaged every two weeks. To prepare for end...
Embodiment 2
[0421] Example 2: Endoderm differentiation using hESCs
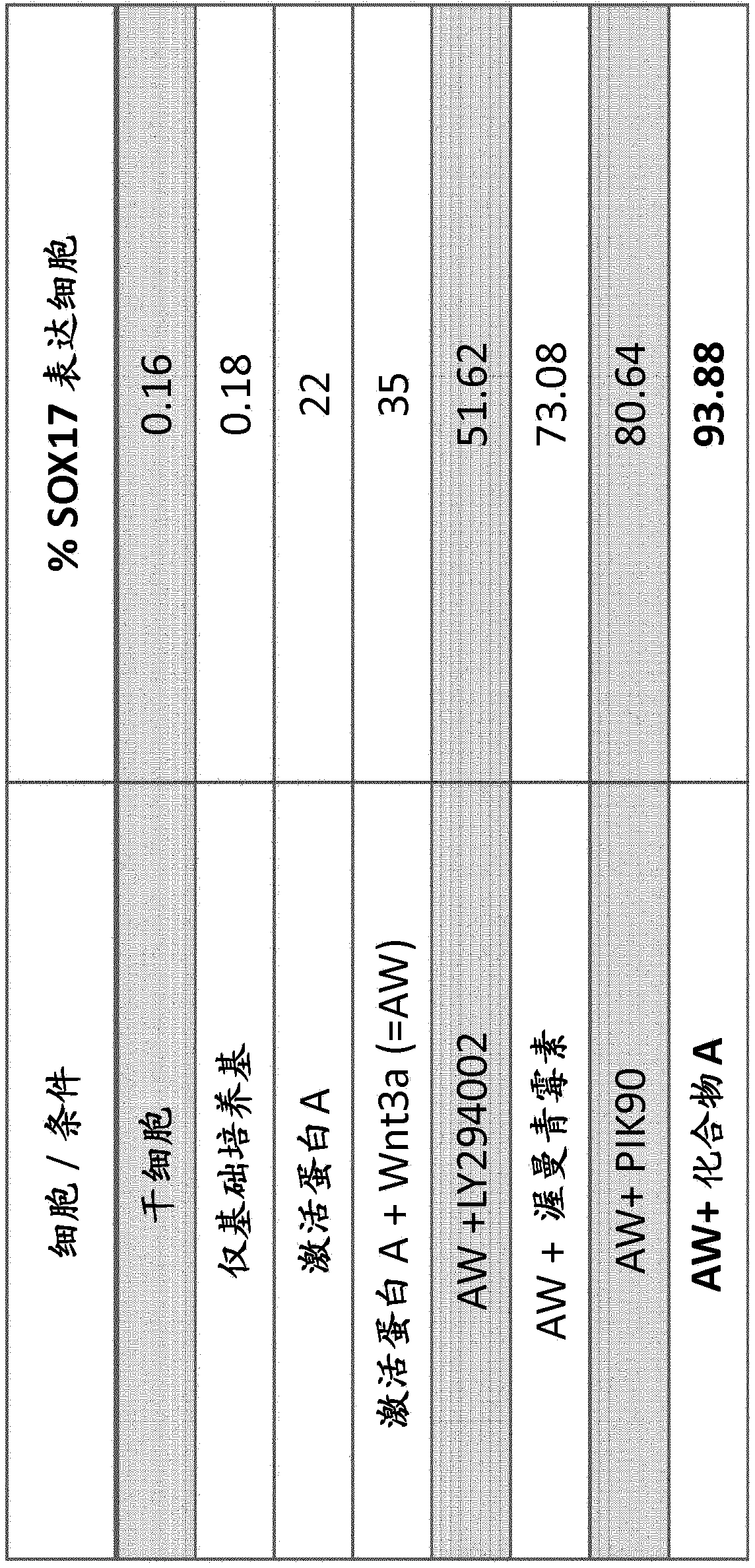
[0422] Comparison of the effects of various commercially available PI3K inhibitors on endoderm differentiation. hESC cell samples were prepared as described above and in basal medium or supplemented with Activin A; Activin A and 50 μg / ml human Wnt3a (R&D, #5036-WN-010); or Activin A, Wnt3A and Table 1 below. Differentiate in basal medium with one of the listed PI3K inhibitors.
[0423] Table 1
[0424] PI3K inhibitors
Concentrations used in Example 2
500nM
Wortmannin
250nM
PIK90
500nM
LY294002
5μM
[0425] Except for Compound A, the compounds shown in Table 1 are not isoform-selective PI3K inhibitors.
[0426] The structure of Compound A is provided below:
[0427]
[0428] After 3 days of treatment, cells were harvested and stained for flow cytometry analysis with anti-SOX17 antibody in preparation as described above. exist figure 1 Th...
Embodiment 3
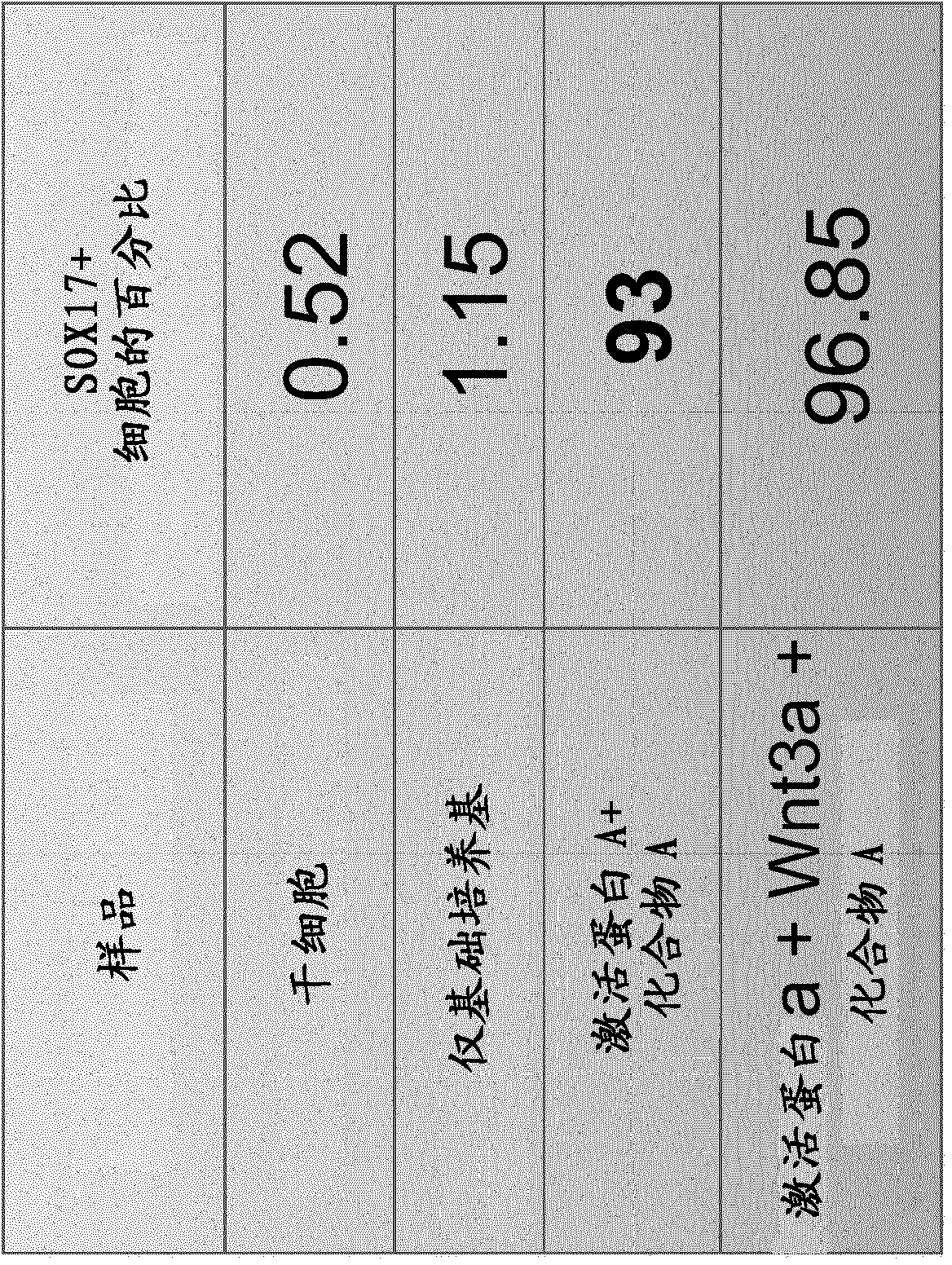
[0429] Example 3: Wnt3a is not essential for differentiation into endoderm
[0430] Experiments were performed to determine whether the growth factor Wnt3a is required for endoderm differentiation. hESC cell samples were prepared as described above and differentiated in basal medium; basal medium supplemented with Activin A, 50 μg / ml Wnt3a and Compound A, or Activin A and 750 nM Compound A alone. After 3 days of treatment, cells were harvested as described above and stained with anti-SOX17 antibody in preparation for flow cytometry analysis. exist figure 2 The results of flow cytometry analysis are shown in . These results indicate that hESC-derived cells cultured with Compound A and Activin A in the absence of Wnt3a convert to endoderm at slightly lower, but comparable, efficiency to cells cultured with Compound A, Activin A and Wnt3a. Such as image 3 As shown in , this effect is independent of the base medium used.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com