Preparation method of iohexol impurity
A technology of iohexol and impurities, applied in the field of medicinal chemistry, can solve the problems of no sales and no public information report (formula 1) compound synthesis method, etc., and achieve the effect of short time consumption, suitable for large-scale production, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
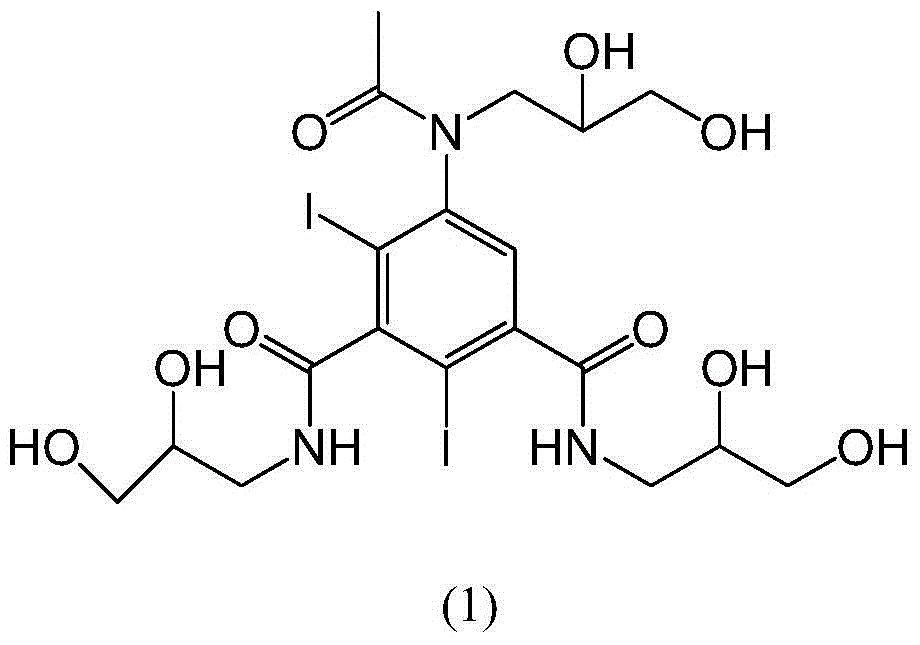
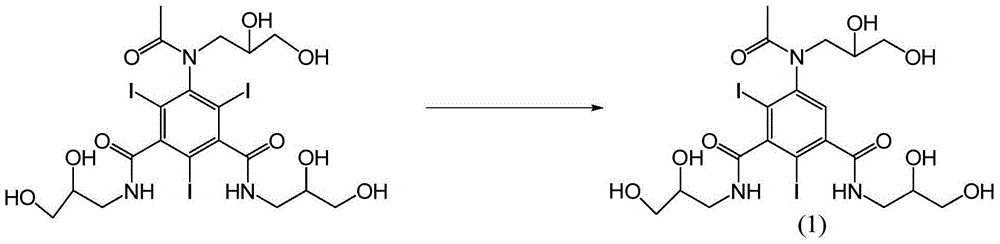
[0032] Example 1: Add 30 g of iohexol into the reaction flask, add water at room temperature and stir to dissolve and clarify. Add 500ml of 0.8% sodium borohydride solution dropwise at 15-35°C, react at 15-35°C until HPLC detection shows that the raw material iohexol is less than 0.1%, then stop the reaction, adjust the pH to 5-7 with hydrochloric acid, water bath Concentrate to dryness under pressure. Add water (120ml) to dissolve, pour the reaction solution into an LX-18 separation resin column with a volume of more than 20 times, absorb for 0.5-2.0 hours, and then elute with purified water. The eluate is detected by HPLC, and the cut-off content is greater than 99.5 % fractions were distilled under reduced pressure to obtain the target product formula (1) as a white solid compound (15.0 g, yield 59.06%, HPLC purity 99.4%). 1 H-NMR (400MHz,D 2 O) δ (ppm) 7.50 (m, 1H), 3.99–3.81 (m, 3H), 3.78–3.21 (m, 12H), 1.78 (s, 3H). 13 C-NMR (100MHz,D 2 O)δ174.4, 174.1, 172.5, 150.2,...
Embodiment 2
[0033] Example 2: Add 30 g of iohexol into the reaction flask, add water at room temperature and stir to dissolve and clarify. Add 300ml of 0.8% potassium borohydride solution dropwise at 15-35°C, and react at 15-35°C until HPLC detection shows that the raw material iohexol is less than 0.1%. Concentrate to dryness under pressure. Add water (60ml) to dissolve it, pour the reaction solution into a separation resin column with a volume of more than 20 times, absorb for 0.5-2.0 hours, and then elute with purified water. The eluate is detected by HPLC, and the fraction with a content greater than 99.5% parts, and distilled under reduced pressure to obtain the target product formula (1) as a white solid compound (12.6 g, yield 49.61%, HPLC purity 99.5%). 1 H-NMR (400MHz,D 2 O) δ (ppm) 7.50 (m, 1H), 3.99–3.81 (m, 3H), 3.78–3.21 (m, 12H), 1.78 (s, 3H). 13 C-NMR (100MHz,D 2 O)δ174.4, 174.1, 172.5, 150.2, 146.5, 144.6, 128.8, 100.6, 90.4, 70.0, 69.7, 68.0, 63.6, 63.4, 51.9, 50.1, 4...
Embodiment 3
[0034] Example 3: Add 30 g of iohexol into the reaction flask, add water at room temperature and stir to dissolve and clarify. Add 100ml of 0.8% potassium borohydride solution dropwise at 15°C, react at 15-35°C until HPLC detection shows that the raw material iohexol is less than 0.1%, then stop the reaction, adjust the pH to 5 with hydrochloric acid, and concentrate to dryness in a water bath under reduced pressure . Add water to dissolve it, pour the reaction solution into a separation resin column with a volume of more than 20 times, absorb for 0.5 hours, and then elute with purified water. The eluate is detected by HPLC, and the fraction with a content greater than 99.5% is intercepted and distilled under reduced pressure. , to obtain the target product formula (1) white solid compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com