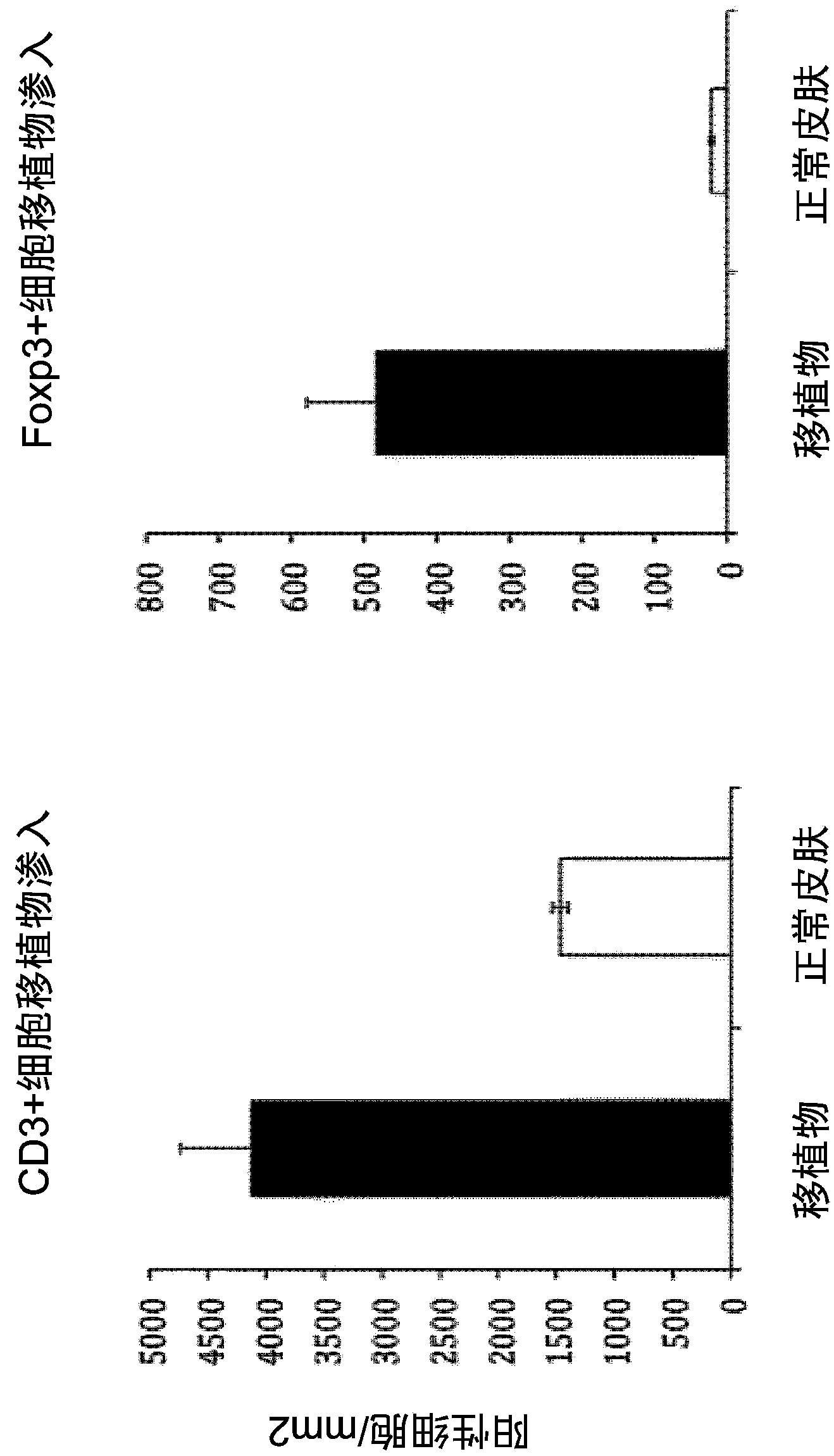
Methods for induction of antigen-specific regulatory t cells
A specific and regulatory technology, applied in animal cells, vertebrate cells, blood/immune system cells, etc., can solve problems that are prone to serious infections and affect the quality of life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0123] Example 1. Induction of apoptosis in vitro
[0124] Antigen Presenting Cells (APCs) Prepared from C57BL / 6 Mice and Loaded with Peptides Covering Class II Restricted T-Cell Epitopes of Autoantigens Involved in Experimental Autoimmune Encephalomyelitis (EAE) for Multiple Sclerosis Model .
[0125] Therefore, with the sequence VGW YRSPFSRVV The myelin oligodendrocyte glycoprotein (MOG) peptide of HLYR [SEQ ID.NO: 1], corresponding to amino acid residues 37-52 of the MOG protein, was used to load the cells.
[0126] This peptide contains dominant T cell epitopes. The P1 position, the first amino acid anchored to the MHC class II groove, is Y40 (P1-P9 sequences are underlined).
[0127] Cytolytic CD4+ T cells (cCD4+ T cells) were obtained from the spleen of animals immunized four times with aluminum hydroxide and 50 μg of the peptide of SEQ ID. NO: 1, wherein the amino-terminal 3 amino acids are replaced by the sequence CGPC, generating the sequence CGPC Peptide of YR...
Embodiment 2
[0129] Example 2. Isolation of apoptosomes
[0130] The supernatant of apoptotic cells obtained in Example 1 was collected and subjected to two centrifugation steps (500×g, 5 minutes) to remove cells. Then, the supernatant was filtered through a 1.2 μM hydrophilic syringe filter. After centrifugation at 100,000 xg for 30 minutes, the apoptotic bodies contained in the pellet were harvested and used for cell experiments.
[0131] Alternatively, apoptotic cells and apoptosomes can be separated by affinity using antibodies directed against cell surface components expressed as a result of apoptosis. Examples of these are antithrombin antibodies. In a preferred preparation step, anti-thrombin antibodies are covalently coupled to magnetic microbeads. After incubation for 1 hour at 20°C with gentle shaking, the beads remained on the magnet. Then, apoptosomes were recovered by elution with a weakly acidic buffer.
[0132] These methods are known in the art. (Schiller et al. (2008...
Embodiment 3
[0133] Example 3. Generation or Obtaining of Immature Dendritic Cells (iDC)
[0134] Bone marrow progenitor cells were obtained from the upper and lower patella. B and T lymphocytes were removed using magnetic reduction using CD19 and CD90 microbeads, respectively. Negative isolates containing CD19-CD90-iDC progenitor cells were resuspended in serum-free medium containing 500 U / ml recombinant GM-CSF and seeded (3x10 6 cells / ml) on tissue culture plates and maintained at 37°C. Cells were washed every other day for 6 days to avoid disrupting aggregates. On day 6, iDC aggregates were removed, washed and added to new plates. On day 7, cells were harvested and used for analysis.
[0135] These methods are known in the art. See eg, Inaba et al. (2009) Curr. Prot. immunol. 1(86), Section 3.7. Unit pp. 10-12.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com