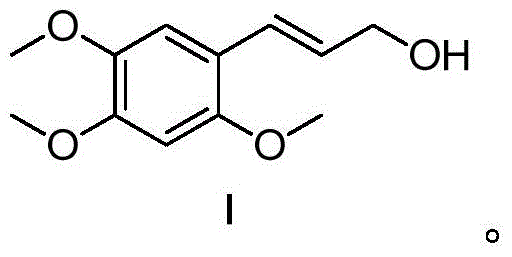
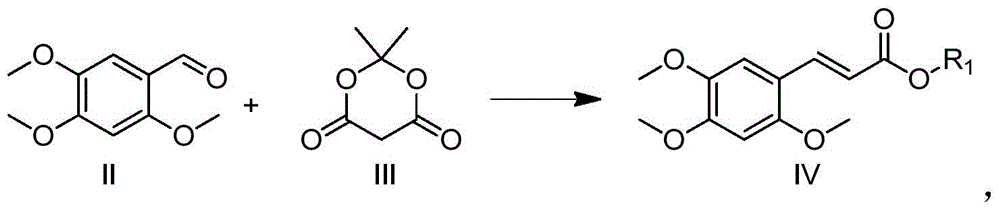
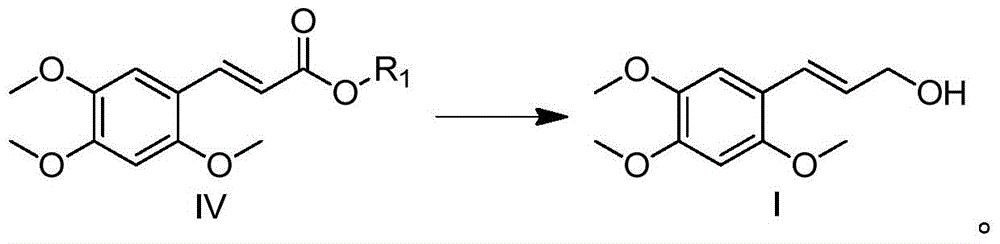
α-Asarocyl alcohol and its preparation method and application
A technology of asaranol and fatty alcohol, applied in the field of preparation of alpha-asaranol, can solve problems such as cognitive dysfunction, severe allergic reaction and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Preparation of Methyl 2,4,5-Trimethoxycinnamate
[0022] A 3L three-necked flask with a thermometer and a condenser tube was added with 195.5g (1.35mol) of McFarlandic acid (1.35mol), methanol (50mL) and toluene (750mL), heated to reflux at 110°C for 4 hours, cooled to room temperature, and then added with 2,4 , 196.2g (1.0mol) of 5-trimethoxybenzaldehyde, 134.5g (1.7mol) of pyridine, and 14.5g (0.17mol) of piperidine, heated to reflux for 18 hours, concentrated under reduced pressure, then added ethyl acetate (200mL), Water (200mL), extracted and separated 3 times, combined the organic phases, concentrated under reduced pressure, added ethanol (1000mL), refrigerated overnight in the refrigerator, after the precipitation was precipitated, filtered with suction, washed 3 times with 500mL of ice ethanol to obtain a light yellow solid 166.3 g, 66% yield.
[0023] m / z=[M+1]253.1086
[0024] 1 H NMR (600MHz, cdcl3) δ7.97(d, J=16.1Hz, 1H), 7.01(s, 1H), 6.50(s, 1H), 6.38(d,...
Embodiment 2
[0027] Preparation of ethyl 2,4,5-trimethoxycinnamate
[0028] In a 250mL three-neck flask with a thermometer and a condenser tube, add 20.2g (0.14mol) of McFarlandic acid (0.14mol), ethanol (10mL), and toluene (150mL) respectively, heat and reflux at 110°C for 4 hours, cool to room temperature, and then add 2,4 , 19.6g (0.1mol) of 5-trimethoxybenzaldehyde, 13.5g (0.17mol) of pyridine, and 1.5g (0.017mol) of piperidine, heated to reflux for 18 hours, concentrated under reduced pressure, then added ethyl acetate (50mL), Water (50mL), extracted and separated 3 times, combined the organic phases, concentrated under reduced pressure, added ethanol (150mL), refrigerated overnight, after the precipitate was precipitated, filtered with suction, washed 3 times with 100mL ice-ethanol to obtain a light yellow solid 16.0 g, 60% yield.
Embodiment 3
[0030] Preparation of Propyl 2,4,5-Trimethoxycinnamate
[0031] In a 250mL three-neck flask with a thermometer and a condenser tube, add 10.1g (70mmol) of McBurney's acid, propanol (8mL), and toluene (80mL) respectively, heat and reflux at 110°C for 4 hours, cool to room temperature, and then add 2,4 , 5-trimethoxybenzaldehyde 9.8g (50mmol), pyridine 6.7g (85mmol), piperidine 0.75g (8.5mmol), heated to reflux for 18 hours, concentrated under reduced pressure, added ethyl acetate (20mL), water ( 30mL), extracted and separated 3 times, combined the organic phases, concentrated under reduced pressure, added ethanol (80mL), refrigerated overnight in the refrigerator, after the precipitation was precipitated, filtered with suction, washed 3 times with 50mL of ice ethanol, and obtained 7.7g of light yellow solid , yield 55%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com