Preparation method of stable protein drug-loaded microparticle system
A technology of drug-loaded particles and proteins, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, anti-tumor drugs, etc., can solve problems such as loss of protein activity, unknown and unsafe product quality, and affect the quality of pharmaceutical products. Effects of mildness, increased stability and practicality, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
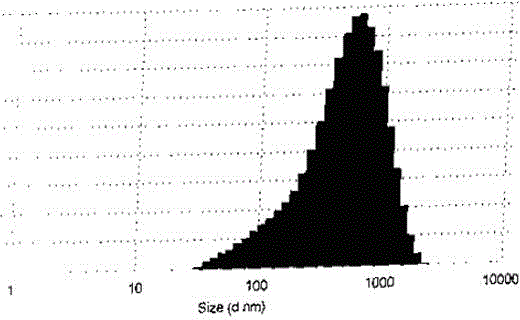
[0032] Dissolve 10mg of mitomycin in 1ml of N,N-dimethylacetamide, then add 1ml of 5% sodium chloride solution and mix well, and finally add 20% human serum albumin solution dissolved in pH 7.8 phosphate buffer 2ml to form a homogeneous solution. At 0°C, the homogeneous solution was directly freeze-dried into a powder injection preparation, and the final powder injection product was diluted with saline, and the particle size was measured by Malvern particle size analyzer ZS90 and the drug loading was determined by HPLC. The results show that the average particle size of the human serum albumin-loaded mitomycin microparticle system is 290.9nm, and the particle size and particle size distribution diagram are shown in figure 1 . The drug loading of microparticles reached 2.1%.
Embodiment 2
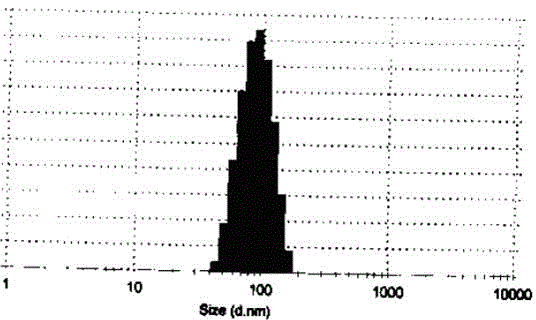
[0034] Dissolve 1 mg of bortezomib in 1 ml of tert-butanol, then add 0.5 ml of 2% sodium chloride solution and mix well, and finally add 0.5 ml of 2% bovine serum albumin solution to form a homogeneous solution. At room temperature, the homogeneous solution was directly freeze-dried into a powder injection preparation, and the final powder injection product was diluted with normal saline, and the particle size was measured with a Malvern particle size analyzer ZS90 and the drug loading of the microparticles was determined by HPLC. The results showed that the prepared microparticle system averaged The particle size is 164nm, and the drug loading capacity of the microparticles reaches 7.8%.
Embodiment 3
[0036] Dissolve 10mg of decitabine in 1ml of dimethyl sulfoxide, then add 1ml of 1% sodium phosphate solution and mix well, and finally add 0.4ml of 50% bovine serum albumin solution dissolved in pH 7.0 phosphate buffer to form a homogeneous phase solution. At room temperature, the homogeneous solution was directly freeze-dried into a powder injection preparation, and the final powder injection product was diluted with normal saline, and the particle size was measured with a Malvern particle size analyzer ZS90 and the drug loading of the microparticles was determined by HPLC. The results showed that the prepared microparticle system averaged The particle size is 264nm, and the drug loading capacity of the microparticles reaches 4.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap