A kind of method for synthesizing silibinin by enzyme-catalyzed reaction
A catalytic reaction and silibinin technology, applied in fermentation and other directions, can solve the problems of long reaction time, achieve rapid enzymatic reaction, simple reaction conditions, and improve safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Verification of peroxidase-catalyzed silibinin synthesis
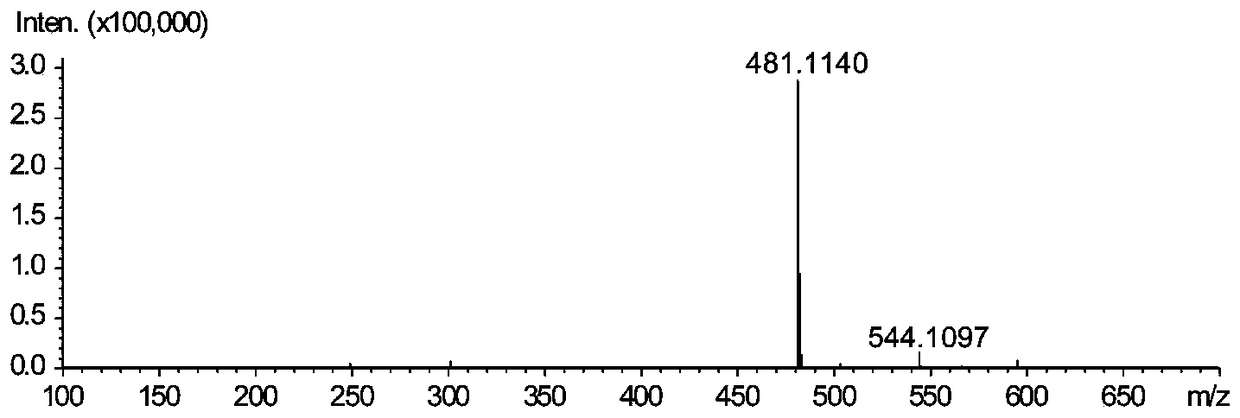
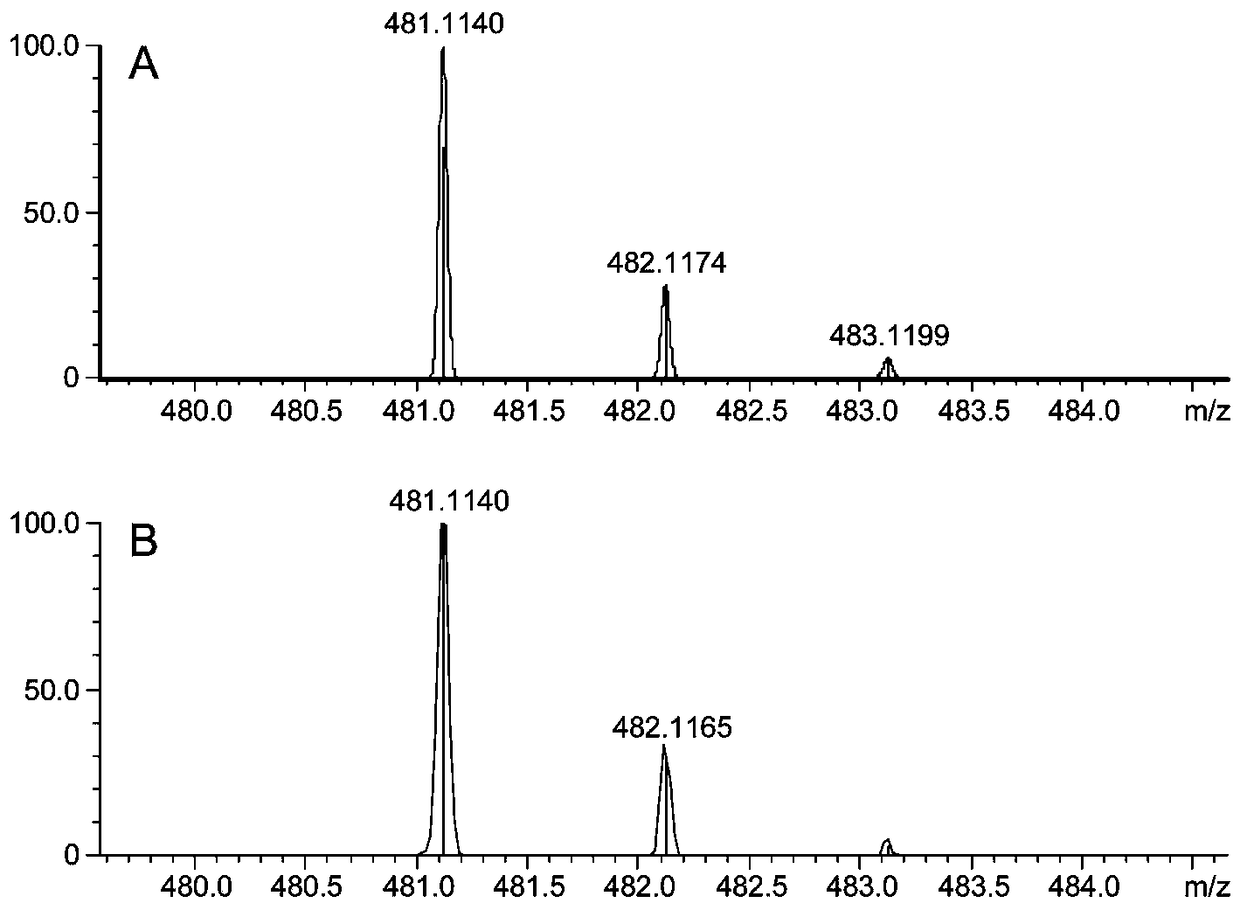
[0031] In order to verify the ability of peroxidase to catalyze the synthesis of silibinin from coniferyl alcohol and docetaxel, coniferyl alcohol and docetaxel were used as substrates in H 2 o 2 In the presence of conditions, the synthesis of silibinin is catalyzed by horseradish peroxidase (HRP). It was verified that the omitted substrate, H 2 o 2 Or enzyme, the production of silybin, as shown in Table 1.
[0032] The chemical reaction formula of the synthetic reaction of silybin is as Image 6 As shown, theoretically, one molecule of H is needed for every molecule of silybin synthesized 2 o 2 Participate in the reaction, when the molar ratio of coniferyl alcohol and docetaxel is 2:1, it is the optimum substrate ratio for the synthesis of silybin. In view of the fact that silibinin will be absorbed by the H in the reaction system at the same time 2 o 2 Therefore, we at first react under the ...
Embodiment 2
[0039] Catalytic synthesis of silibinin under the insufficient condition of embodiment 2 hydrogen peroxide
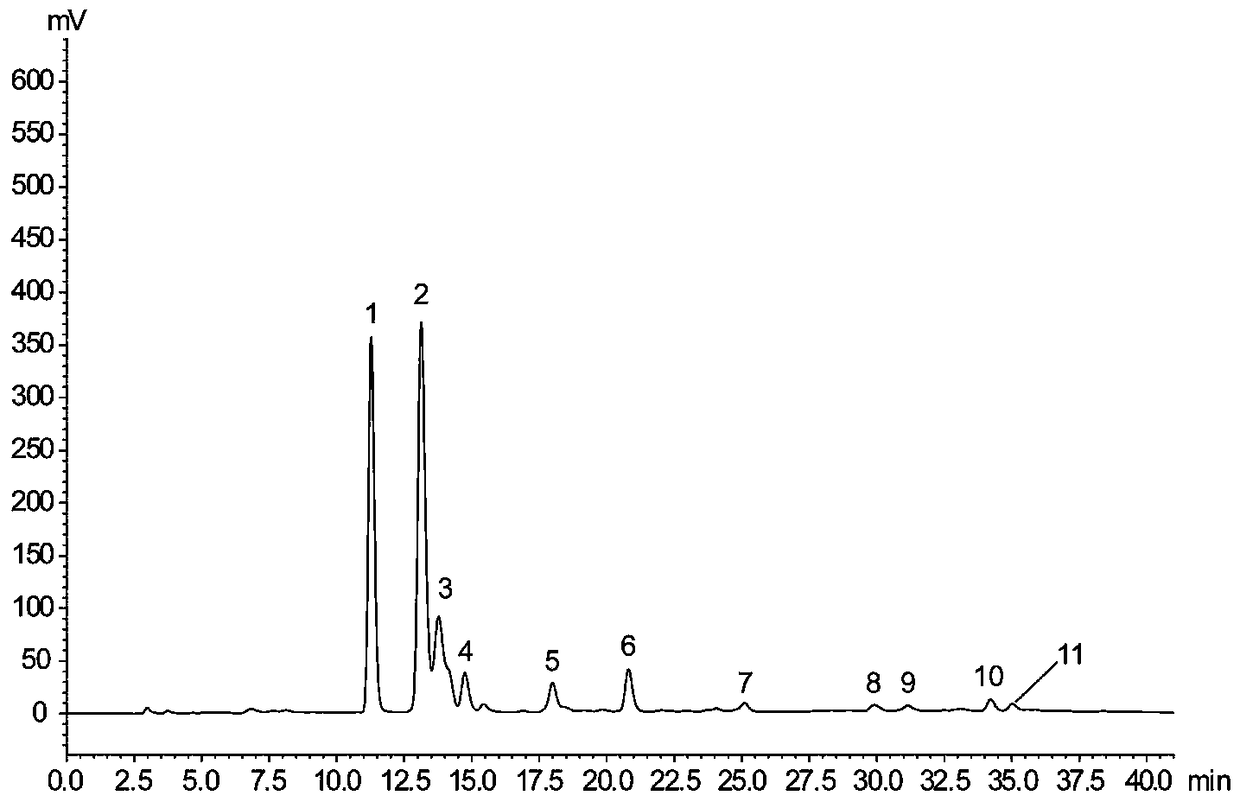
[0040] In the presence of hydrogen peroxide, peroxidase can catalyze the synthesis of silibinin; at the same time, silybin can be decomposed by hydrogen peroxide in the reaction system. Therefore, the reaction is actually a dynamic equilibrium process. In this embodiment, the synthesis reaction of silibinin is carried out under the condition of insufficient hydrogen peroxide. Same as Example 1, react according to the conditions of molar ratio coniferyl alcohol:docetaxel=2:1, and insufficient hydrogen peroxide (about 45.3% of docuscetaxel), specifically as shown in table 2. All reactions were carried out 3 times and the average value was taken.
[0041] Table 2 Reaction system under the condition of insufficient hydrogen peroxide
[0042]
[0043] *MeOH is added after the reaction to terminate the enzymatic reaction.
[0044] We studied the change of silibinin pro...
Embodiment 3
[0047] Catalytic synthesis of silibinin under the condition of sufficient amount of hydrogen peroxide in embodiment 3
[0048] In the present embodiment, hydrogen peroxide is equal to the amount of docetaxel, and the mol ratio of coniferyl alcohol and docetaxel is still 2:1, that is, coniferyl alcohol: docetaxel: H 2 o 2 =2:1:1.
[0049] Dilute 30% hydrogen peroxide to 12.23mM with PP Buffer (pH7.0). Dissolve horseradish peroxidase at 5000 U / mL in PP Buffer (pH 7.0). Other reagent concentration is identical with embodiment 1. The specific reaction system is shown in Table 3. The reaction times are: 2, 5, 10, 15, 20, 30, 45, 60 min. All reactions were carried out 3 times and the average value was taken.
[0050] Table 3 Reaction system under the condition of sufficient hydrogen peroxide
[0051]
[0052]
[0053] *MeOH is added after the reaction to terminate the enzymatic reaction.
[0054] The results show that when there is enough hydrogen peroxide to participa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com