Production method for composite lactose for injection
A production method and technology for injection, which are applied in the field of medicine, can solve the problems of not meeting the requirements of injections, insufficient freeze-drying protection efficiency, and low purity of lactose, and achieve a simple and feasible purification process, improve freeze-drying protection efficiency, and be environmentally friendly. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
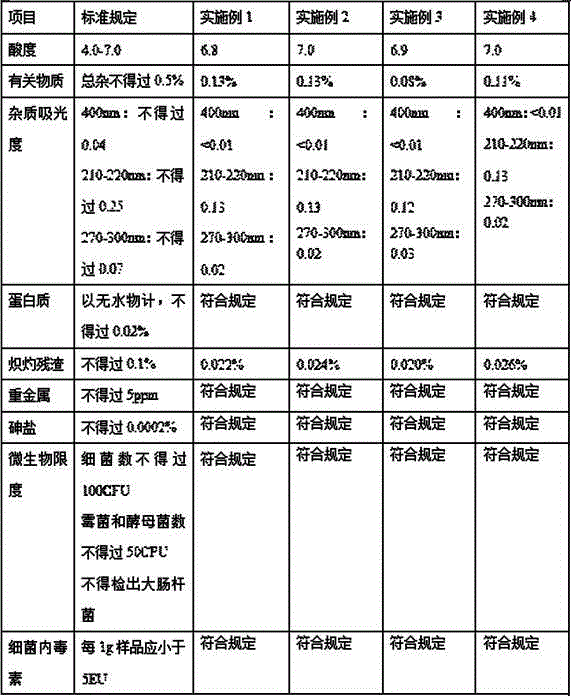
Embodiment 1
[0025] 1. Weigh 100g of crude lactose, add 400ml of purified water, stir and heat to 30°C, make it completely dissolve to form a lactose solution;
[0026] 2. Filter the crude lactose solution with an ultrafiltration column with a molecular weight cut-off of 3000MW;
[0027] 3. Perform nanofiltration on the ultrafiltered lactose solution;
[0028] 4. Add the filtered lactose into 150ml of purified water, stir and heat to 70°C to completely dissolve into a lactose solution;
[0029] 5. Add 450ml of ethanol to the lactose solution in step 4, stir and cool to 10°C to precipitate crystals, and keep this process for 0.5 hours;
[0030] 6. Centrifuge the solution with crystals in a filter bag centrifuge to collect the crystals;
[0031] 7. Take 99 grams of crystals and 100 ml of 1% hydroxypropyl methylcellulose solution, mix well, and then spray dry.
Embodiment 2
[0033] 1. Weigh 20Kg of crude lactose, add 62L of purified water, stir and heat to 40°C, make it completely dissolve to form a lactose solution;
[0034] 2. Filter the crude lactose solution with an ultrafiltration column with a molecular weight cut-off of 3000MW;
[0035] 3. Perform nanofiltration on the ultrafiltered lactose solution;
[0036] 4. Add the filtered lactose into 30L of purified water, stir and heat to 70°C to completely dissolve into a lactose solution;
[0037] 5. Add 100L of ethanol to the lactose solution in step 4, stir and cool to 10°C to precipitate crystals, and keep this process for 1 hour;
[0038] 6. Centrifuge the solution with crystals in a filter bag centrifuge to collect the crystals;
[0039] 7. Take 97 grams of crystals and 100ml of 3% hydroxypropyl methylcellulose solution, mix well and then spray dry.
Embodiment 3
[0041] 1. Weigh 20Kg of crude lactose, add 62L of purified water, stir and heat to 35°C to completely dissolve to form a lactose solution;
[0042] 2. Filter the crude lactose solution with an ultrafiltration column with a molecular weight cut-off of 3000MW;
[0043] 3. Perform nanofiltration on the ultrafiltered lactose solution;
[0044] 4. Add the filtered lactose into 32L of purified water, stir and heat to 70°C to completely dissolve into a lactose solution;
[0045] 5. Add 100L of ethanol to the lactose solution in step 4, stir and cool to 10°C to precipitate crystals, and keep this process for 1 hour;
[0046] 6. Centrifuge the solution with crystals in a filter bag centrifuge to collect the crystals;
[0047] 7. Take 98g of crystals and 100ml of 2% hydroxypropyl methylcellulose solution, mix well, and then spray dry.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com