Preparation method of urea compound
A technology of compound and mixed solution, which is applied in the field of preparation of urea compounds, can solve the problems of affecting the reaction, easy to decompose, cumbersome operation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
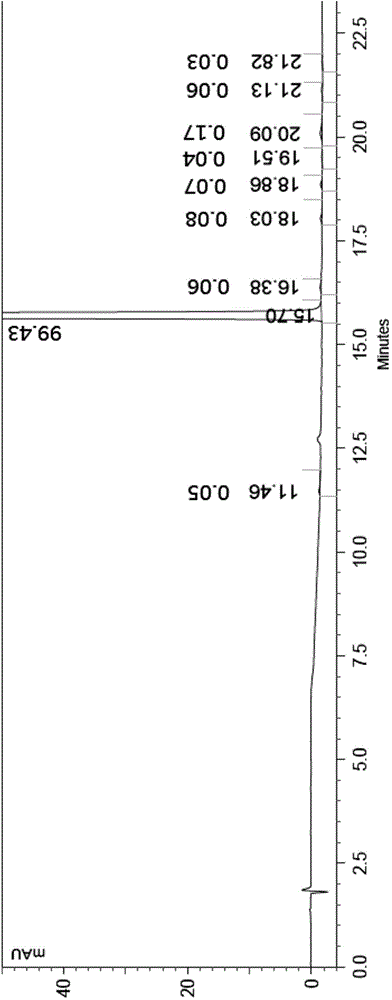
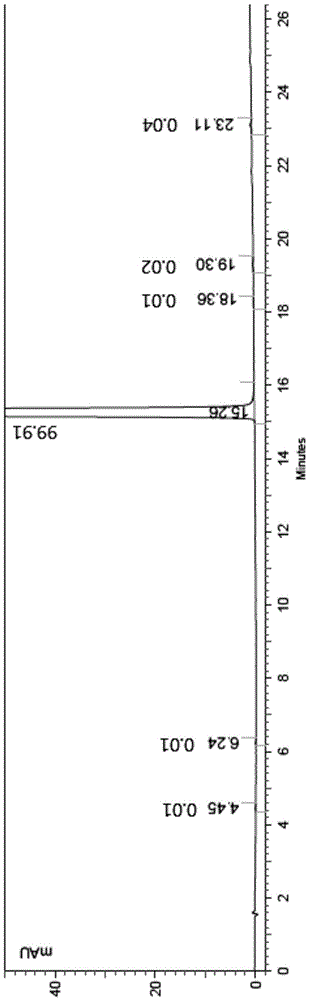
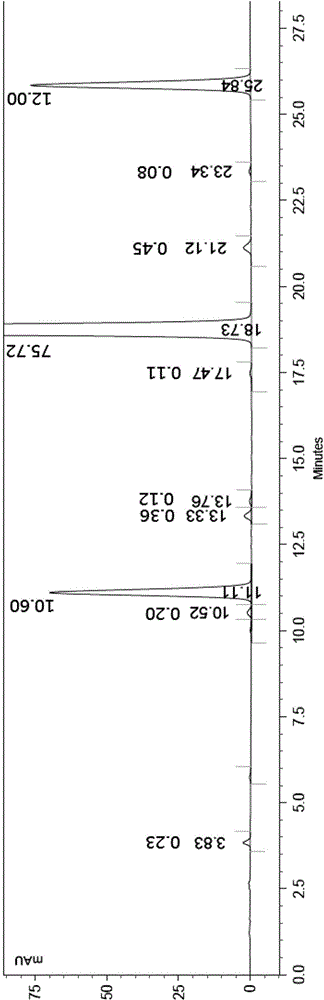
Image
Examples
Embodiment Construction
[0007] The contriver finds through research, uses N-methyl-4-chloro-2-pyridinecarboxamide to react with 4-aminophenol in the presence of a strong base, and it is easy to make the resulting product 4-(4-aminophenoxy)-N -Methyl-2-pyridinecarboxamide contains a small part of base, and the existence of this small part of base makes 4-(4-aminophenoxy)-N-methyl-2-pyridinecarboxamide and 4-chloro- 3-trifluoromethyl-phenylisocyanate generates a symmetrical urea impurity in the reaction process, its structure is shown in the following formula (A), it is difficult to remove from Sorafenib, affecting the quality of the target product Sorafenib and yield; and the compound 4-(4-aminophenoxy)-N-methyl-2-pyridinecarboxamide solid is mixed with water, treated, and then with 4-chloro-3-trifluoromethyl- The phenylisocyanate reaction can avoid a large amount of production of the impurity (A), and can obtain the product Sorafenib with high yield and high purity;
[0008]
[0009] On the one h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com