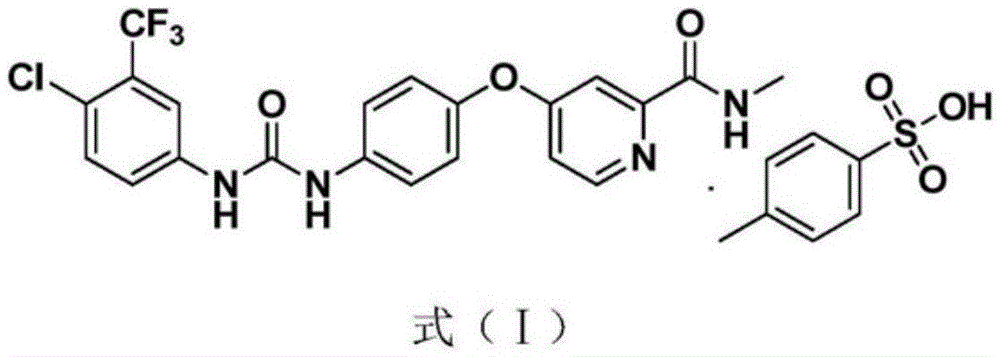
Sorafenib compound
A sorafenib and compound technology, applied in the field of drug preparation, can solve problems such as low yield and purity, long reaction time, and inconvenient operation, and achieve the effects of lower reaction temperature, shorter reaction time, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] Solid base carrier Al 2 o 3 Preparation of / KOH:
[0042] At room temperature, make 20 grams of KOH into a saturated aqueous solution, and add 60 grams of neutral Al 2 o 3 (100-200 mesh) stirred and reacted at 70°C for 1.5 hours, then evaporated to dryness, and dried the solid base at 120°C to constant weight to obtain the solid base carrier Al 2 o 3 / KOH.
[0043] Solid base carrier Al 2 o 3 Preparation of / KF:
[0044] Dissolve 58 grams of KF in 100ml of water, add 100 grams of neutral Al 2 o 3 (100-200 mesh) stirred and reacted at 65°C for 1 hour, evaporated to dryness, and dried the solid base at 120°C for 4 hours to obtain the solid base carrier Al 2 o 3 / KF.
[0045] Composite solid base Al 2 o 3 / KOH / K 2 CO 3 Preparation of:
[0046] At room temperature, 28 grams of KOH, 69 grams of K 2 CO 3 Prepare saturated aqueous solutions respectively, after mixing the above two protective aqueous solutions, add 101 grams of neutral Al 2 o 3 (100-200 mes...
Embodiment 1
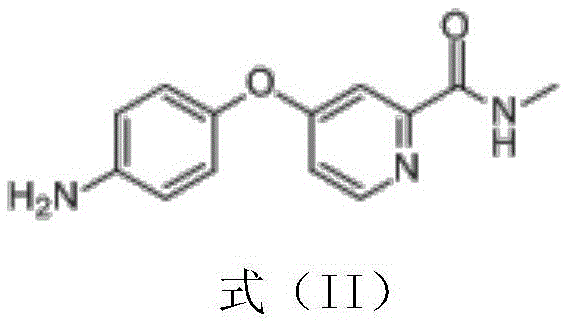
[0060] 1) Preparation of crude product of 4-(4-aminophenoxy)-N-methyl-2-pyridinecarboxamide
[0061] 4-Chloro-N-methylpyridine-2-carboxamide (170.6g, 1mol) and p-aminophenol (120g, 1.1mol) were stirred and dissolved in 853ml of acetonitrile, and 24.4g of phase transfer catalyst 18-crown- 6 and composite solid base carrier Al 2 o 3 / KOH / K 2 CO 3 1365g, heat to reflux for 1.5h after the reaction is complete, add 4.9g of filter aid diatomaceous earth for hot filtration, and then concentrate to obtain a light yellow powder.
[0062] 2) Recrystallization
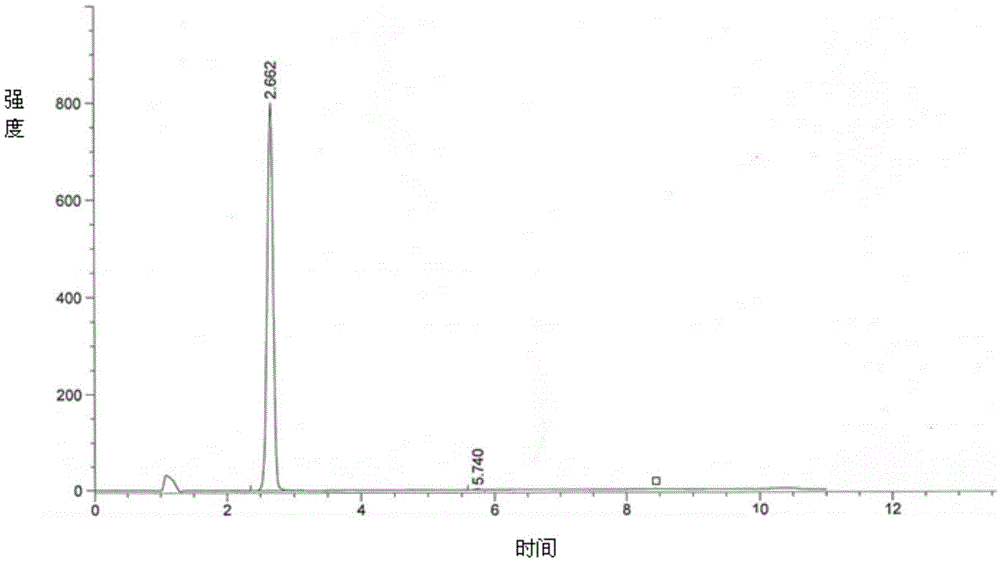
[0063] Add the light yellow powder obtained in step 1) into 853ml of ethyl acetate, heat to reflux with stirring, continue to reflux for 30-40 minutes after dissolving, cool down to 25-35°C, keep stirring and wash the crystal for 3-8 hours, centrifuge The obtained wet product is dried at 45-50° C. for 10-15 hours to obtain the pure product of 4-(4-aminophenoxy)-N-methyl-2-pyridinecarboxamide. Yield 92.5%, purity 99.8%, see ...
Embodiment 2
[0065] 1) Preparation of crude product of 4-(4-aminophenoxy)-N-methyl-2-pyridinecarboxamide
[0066] 4-Chloro-N-methylpyridine-2-carboxamide (85.3g, 0.5mol) and p-aminophenol (54.6g, 0.5mol) were stirred and dissolved in DMF512ml, and a phase transfer catalyst tetrabutylammonium bisulfate was added (TBAB, 6.8g) and composite solid base support Al 2 o 3 / NaOH / K 2 CO 3 597g, react at room temperature for 1.8h After the reaction is complete, add 3.4g of filter aid perlite for hot filtration, and then concentrate to obtain a light yellow powder.
[0067] 2) Recrystallization
[0068] Add the light yellow powder obtained in step 1) into 512ml of isopropanol, heat to reflux under stirring conditions, continue to reflux for 30-40 minutes after dissolving, cool down to 25-35°C, keep stirring and wash the crystal for 3-8 hours, centrifuge The obtained wet product is dried at 45-50° C. for 10-15 hours to obtain the pure product of 4-(4-aminophenoxy)-N-methyl-2-pyridinecarboxamide. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com