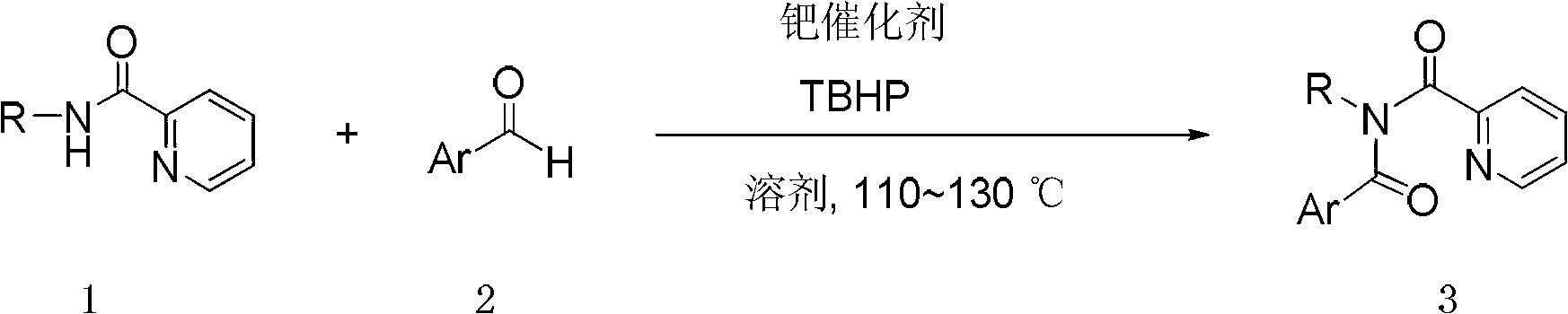
Preparation method of para-diimide derivative
A technology for secondary diimides and derivatives, which is applied in the field of preparation of secondary diimide derivatives, can solve the problems of reducing the reactivity of nitrogen-hydrogen bonds on amides, large steric hindrance, etc., and achieve saving of reaction steps and operation The effect of simplicity and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
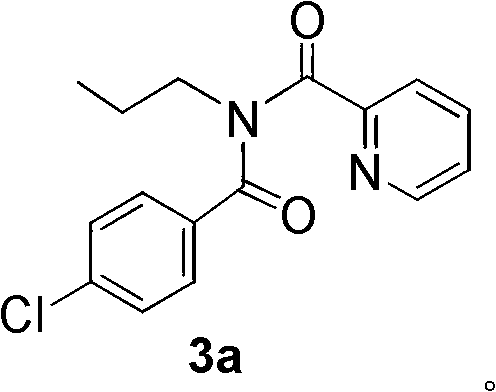
[0038] In this example, N-propyl-N-(4-chlorobenzoyl)pyridinecarboxamide 3a was prepared by the carbon-hydrogen functionalization reaction of N-propylpicolinamide and 4-chlorobenzaldehyde.
[0039] A solution of N-propylpicolinamide (0.25mmol), 4-chlorobenzaldehyde (0.5mmol) and bis(triphenylphosphine)palladium acetate (0.025mmol, 10mol%) in acetonitrile (1.0mL) was added at room temperature After stirring for 2 minutes, a decane solution (5.5 M) of tert-butanol peroxide (4.0 mmol) was added dropwise. The reaction mixture was stirred at 120° C. for 24 hours to complete the reaction. Concentrate the mixed solution, use petroleum ether / ethyl acetate=4 / 1 (v / v) mixed solvent as eluent, and through silica gel column chromatography, the desired N-propyl-N-(4-chloro Benzoyl)pyridinecarboxamide 3a in 80% yield.
[0040] The NMR data are as follows: 1 H NMR (300MHz, CDCl 3 ) δ (ppm): 8.30 (d, J = 4.5Hz, 1H), 7.72 (d, J = 7.8Hz, 1H), 7.61 (td, J = 7.5, 1.2Hz, 1H), 7.45 (d, J = 8.7Hz...
Embodiment 2
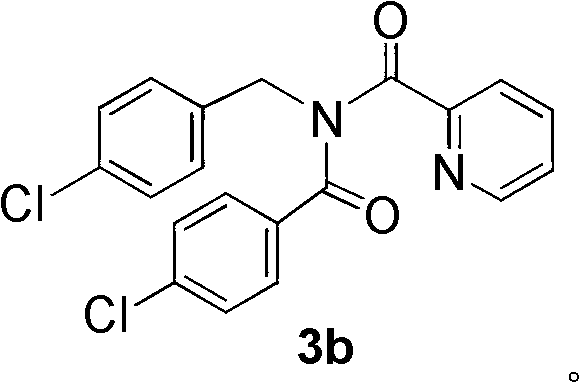
[0044] This example prepares N-(4-chlorobenzyl)-N-(4-chlorobenzoyl) through the carbon-hydrogen functionalization reaction of N-(4-chlorobenzyl)pyridinecarboxamide and 4-chlorobenzaldehyde Pyridinecarboxamide 3b:
[0045] Toluene (1.0mL ) solution was stirred at room temperature for 2 minutes, and a decane solution (5.5M) of tert-butanol peroxide (4.0 mmol) was added dropwise. The reaction mixture was stirred at 120°C for 19 hours to complete the reaction. Concentrate the mixed solution, use petroleum ether / ethyl acetate=8 / 1 (v / v) mixed solvent as eluent, and use silica gel column chromatography to obtain the desired N-(4-chlorobenzyl)-N -(4-Chlorobenzoyl)picolinamide 3b, 74% yield.
[0046] The NMR data are as follows: 1 H NMR (300MHz, CDCl 3 )δ (ppm): 8.30 (d, J = 4.8Hz, 1H), 7.72 (d, J = 7.8Hz, 1H), 7.59 (td, J = 7.5, 1.2Hz, 1H), 7.48 (d, J = 8.1Hz, 2H), 7.39-7.35(m, 2H), 7.28-7.26(m, 2H), 7.15-7.07(m, 3H), 5.19(s, 2H).
[0047] The mass spectrometry data is as follo...
Embodiment 3
[0050] This example prepares N-(3-methoxypropyl)-N-(4-chloro Benzoyl) pyridinecarboxamide 3c:
[0051] N-(3-methoxypropyl)pyridinecarboxamide (0.50mmol)), 4-chlorobenzaldehyde (1.0mmol) and bis(triphenylphosphine)palladium chloride (0.05mmol, 10mol%) in acetonitrile (2.0 mL) was stirred at room temperature for 2 minutes, and a decane solution (5.5 M) of tert-butanol peroxide (8.0 mmol) was added dropwise. The reaction mixture was stirred at 120° C. for 27 hours to complete the reaction. Concentrate the mixed solution, use petroleum ether / ethyl acetate=3 / 1 (v / v) mixed solvent as eluent, and use silica gel column chromatography to obtain the desired N-(3-methoxypropyl) -N-(4-Chlorobenzoyl)pyridinecarboxamide 3c, yield 78%.
[0052] The NMR data are as follows: 1 H NMR (300MHz, CDCl 3 )δ (ppm): 8.30 (d, J = 4.8Hz, 1H), 7.73 (d, J = 7.8Hz, 1H), 7.62 (td, J = 7.8, 1.5Hz, 1H), 7.47 (d, J = 8.4Hz, 2H), 7.18-7.12(m, 3H), 4.13(t, J=7.2Hz, 2H), 3.51(t, J=6.0Hz, 2H), 3.26(s, 3H), 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com