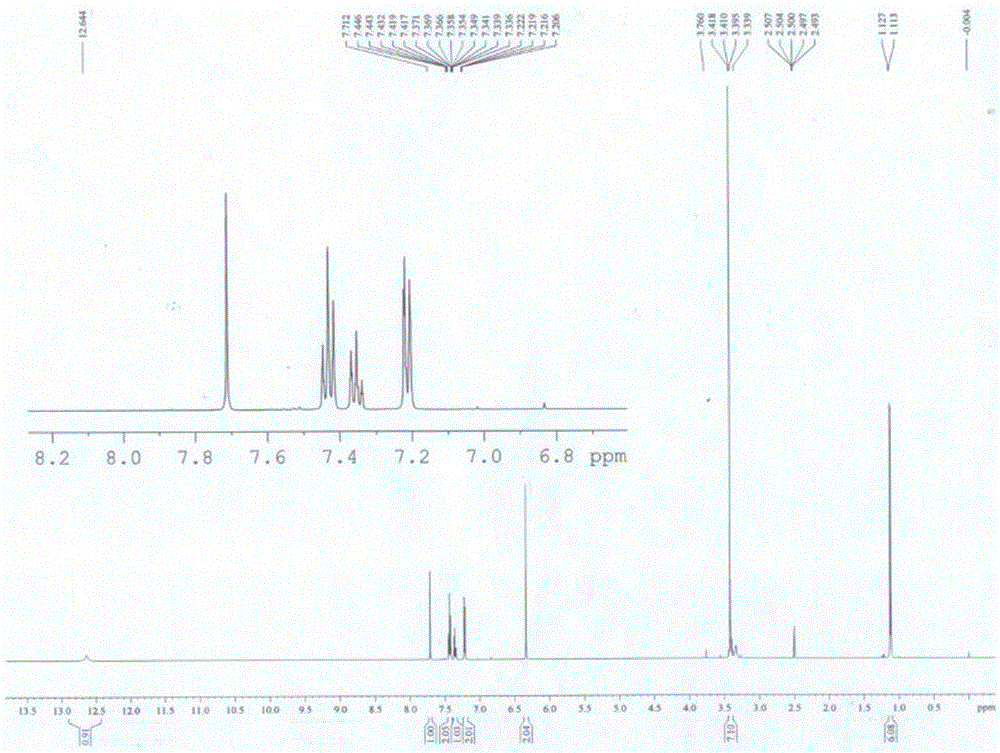
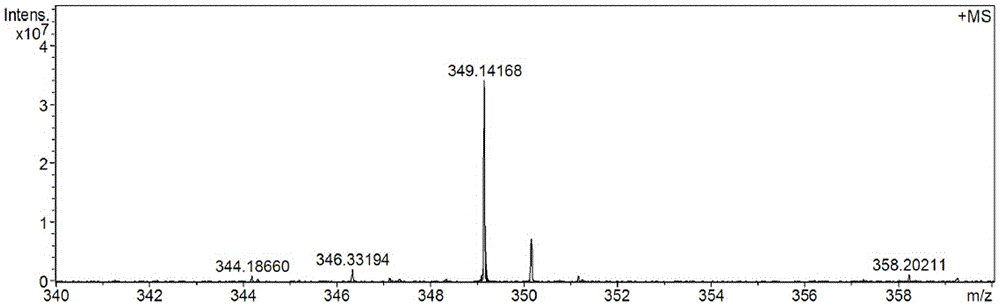
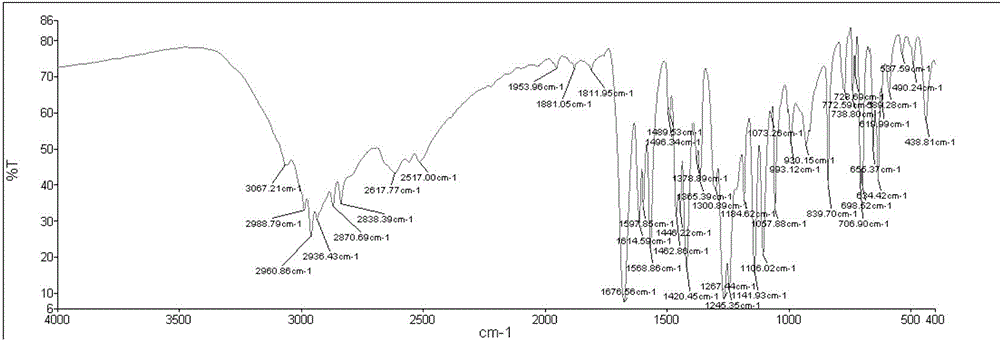
(E) -2-phenyl-3- (3,5-di oxygen-4-isopropine) Puritic acid method
A technology of dimethoxy, cumene, applied in the field of pharmacy, can solve problems such as inability to achieve mass production, inability to obtain purity, and difficulty in separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0042] Example 1 A purification method of (E)-2-phenyl-3 (3,5-dimethoxy-4-isopropylbenzene) acrylic acid
[0043] A method for purifying (E)-2-phenyl-3 (3,5-dimethoxy-4-isopropylbenzene) acrylic acid, which comprises two steps:
[0044] The first step is to selectively esterify (E)-2-phenyl-3(3,5-dimethoxy-4-isopropylbenzene)acrylic acid with a purity of 80% to obtain a purity of 90% % of (E)-2-phenyl-3(3,5-dimethoxy-4-isopropylphenyl)acrylic acid a; react according to the following equation:
[0045]
[0046] Follow the steps below in order:
[0047] 10 g of crude (E)-2-phenyl-3 (3,5-dimethoxy-4-isopropylbenzene) acrylic acid with a purity of 80% (its HPLC diagram is shown in Figure 5 Shown) was added to 60mL of isopropanol and stirred to dissolve, then 0.1g of concentrated sulfuric acid was added dropwise, and the temperature was raised to 60°C. After reacting for 5 hours, cooled to room temperature, the alcohol solvent isopropanol was removed, and ether and saturated ...
Embodiment 2-6
[0056] The purification method of embodiment 2-6 (E)-2-phenyl-3 (3,5-dimethoxy-4-isopropylbenzene) acrylic acid
[0057] Examples 2-6 are respectively a purification method of (E)-2-phenyl-3(3,5-dimethoxy-4-isopropylbenzene)acrylic acid, similar to the purification method of Example 1, The only difference is that the technical parameters involved are different, and the specific differences are shown in the following table:
[0058]
[0059] Note: Substance A in the above table represents (E)-2-phenyl-3(3,5-dimethoxy-4-isopropylphenyl)acrylic acid; substance Aa represents (E)-2-phenyl- 3(3,5-Dimethoxy-4-isopropylphenyl)acrylic acid a; Substance A product represents the final (E)-2-phenyl-3(3,5-dimethoxy-4 - cumene) acrylic products.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com