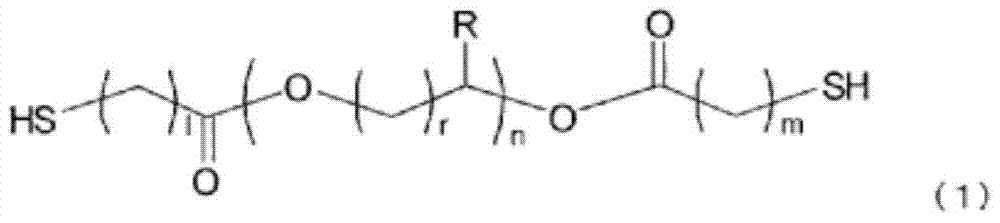
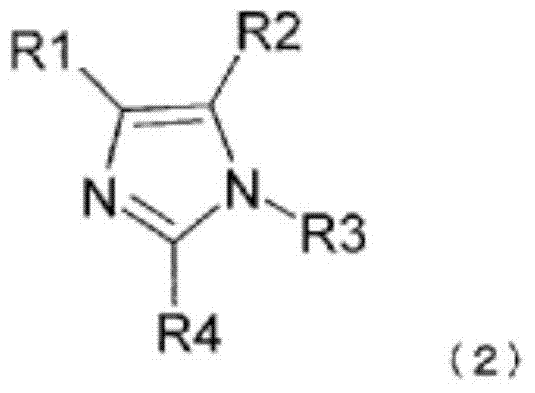
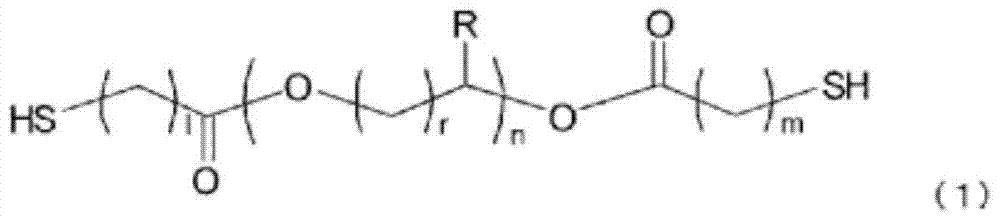
Polymerizable composition, optical material and manufacturing method for same
A technology of polymeric composition and compound, which is applied in optics, optical elements, optical parts, etc., can solve the problems of using imidazoles that have not been specifically disclosed, and achieve the effect of excellent optical physical properties and striae suppression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0166] 2.00 g of catalyst 1,2-dimethylimidazole was added to 8.00 g of internal mold release agent Zelec UN (acid phosphate: registered trademark, manufactured by Stepan Co., Ltd.), and mixed and dissolved at room temperature until a uniform solution was obtained to prepare a catalyst / release agent. Molding agent masterbatch liquid. Add 49.50 g of bis(4-isocyanatocyclohexyl)methane, 8.74 g of 2,5(6)-bis(isocyanatomethyl)-bicyclo[2.2.1]heptane, and further add 1.50 g of Biosorb 583 as an ultraviolet absorber and 0.18 g of the previously prepared catalyst / release agent masterbatch liquid were mixed and dissolved at 20°C. After dissolution, add 37.59 g of 4-mercaptomethyl-1,8-dimercapto-3,6-dithiaoctane and 4.17 g of diethylene glycol dimercaptopropionate, and mix and dissolve at 20°C , to make a homogeneous solution. This homogeneous solution was defoamed at 150 Pa for 30 minutes, filtered with a 1 μm Teflon (registered trademark) filter, and poured into a mold formed of a gla...
Embodiment 2
[0168] 3.33 g of the catalyst 1-benzyl-2-methylimidazole was added to 6.67 g of the internal mold release agent Zelec UN, and mixed and dissolved at room temperature until a uniform solution was obtained to prepare a catalyst / release agent masterbatch liquid. Add 49.50 g of bis(4-isocyanatocyclohexyl)methane and 8.74 g of 2,5(6)-bis(isocyanatomethyl)-bicyclo[2.2.1]heptane, and further add 1.50 g Biosorb 583 as an ultraviolet absorber and 0.15 g of the previously prepared catalyst / release agent masterbatch liquid were mixed and dissolved at 20° C., except that the same method as that described in Example 1 was used. Polymerized, a lens was obtained. The performance evaluation of the obtained lenses is summarized in Table-1.
Embodiment 3
[0170] Add 1.00 g of catalyst 1,2-dimethylimidazole and 1.66 g of 1-benzyl-2-methylimidazole to the internal release agent Zelec UN7.34 g, mix and dissolve at room temperature until it becomes a uniform solution, and prepare catalyst / Release agent masterbatch liquid. Add 49.50 g of bis(4-isocyanatocyclohexyl)methane and 8.74 g of 2,5(6)-bis(isocyanatomethyl)-bicyclo[2.2.1]heptane, and further add 1.50 g Biosorb 583 as an ultraviolet absorber and 0.25 g of the previously prepared catalyst / release agent masterbatch liquid were mixed and dissolved at 20° C., except that the same method as that described in Example 1 was used. Polymerized, a lens was obtained. The performance evaluation of the obtained lenses is summarized in Table-1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com