Albumin-containing pharmaceutical composition and application thereof
A technology of albumin and composition, which is applied in the field of albumin and pharmaceutical composition containing albumin, which can solve problems such as reaction, inability to develop at will, death, etc., to reduce urinary protein, reduce renal function damage, and reduce The effect of glomerulosclerostinuria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: the preparation of denatured bovine blood albumin
[0044]Weigh 10g of bovine serum albumin, add deionized water to dissolve it and make it to 100mL, add 0.45mL of 38% w / v formaldehyde, shake well, let it stand for 30 minutes, add 1mmol / L sodium hydroxide to adjust the pH to 8.9 . Place at 113°C for 1 hour, then at 4°C for 6 weeks, and finally at 100°C for 1.5 hours. Then, 0.9 g of sodium chloride was added, and then 1 mmol / L hydrochloric acid was added to adjust the pH to 7.6. After sterilization, place it in a water bath at 100°C for 30 minutes to obtain denatured bovine serum albumin (the yield is about 100%).
Embodiment 2
[0045] Embodiment 2: the preparation of denatured porcine serum albumin
[0046] Weigh 10g of porcine serum albumin, add deionized water to dissolve it and make it to 100mL, add 0.45mL of 38% w / v formaldehyde, shake well, let stand for 30 minutes, add 1mmol / L sodium hydroxide to adjust the pH to 8.9 . Place at 113°C for 1 hour, then at 4°C for 6 weeks, and finally at 100°C for 1.5 hours. Then, 0.9 g of sodium chloride was added, and then 1 mmol / L hydrochloric acid was added to adjust the pH to 7.6. After sterilization, place it in a water bath at 100°C for 30 minutes to obtain denatured porcine serum albumin (the yield is about 100%).
Embodiment 3
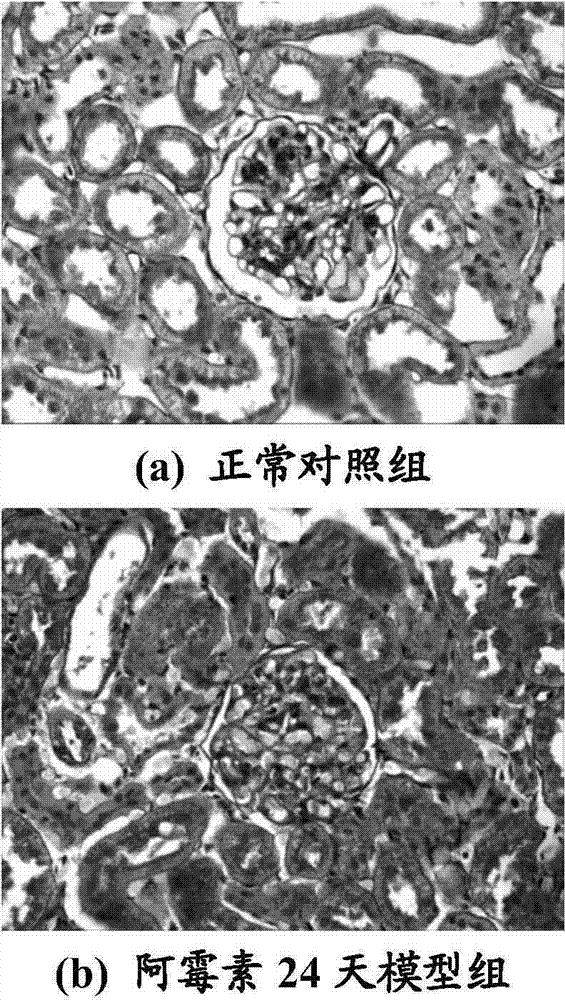
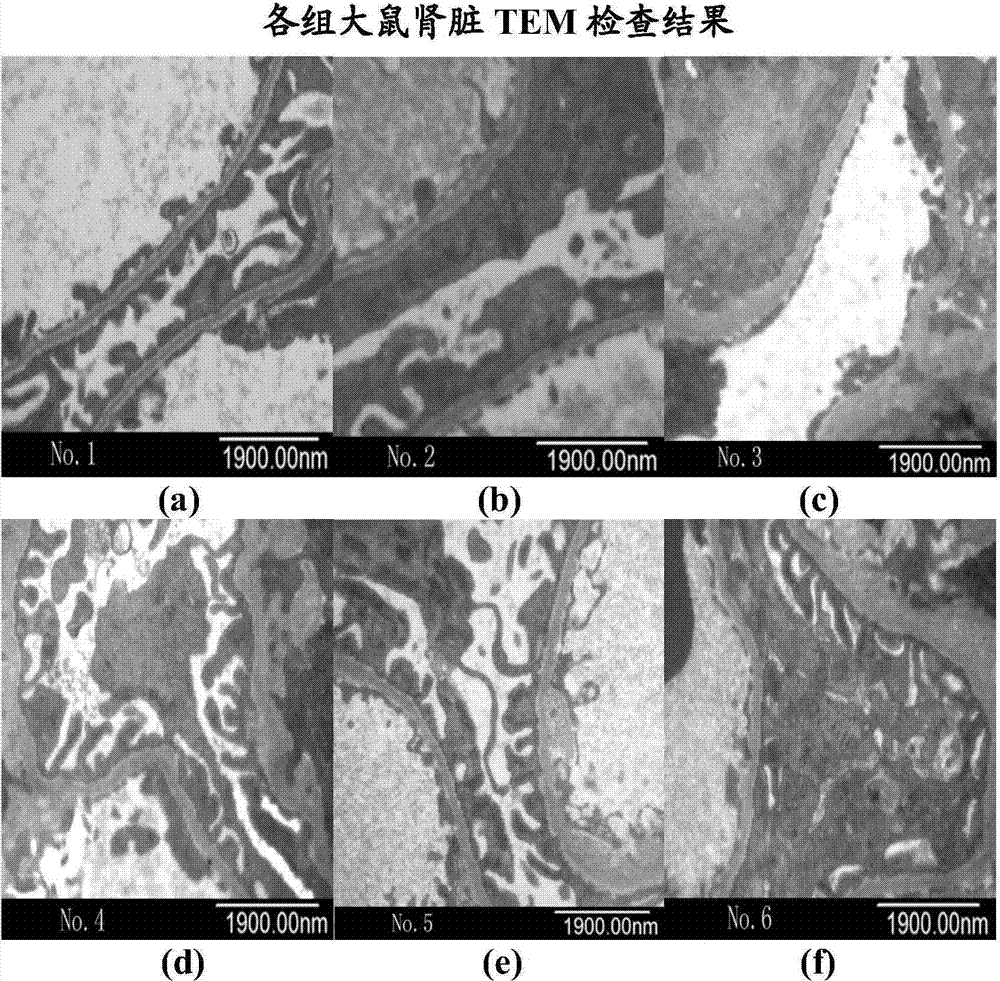
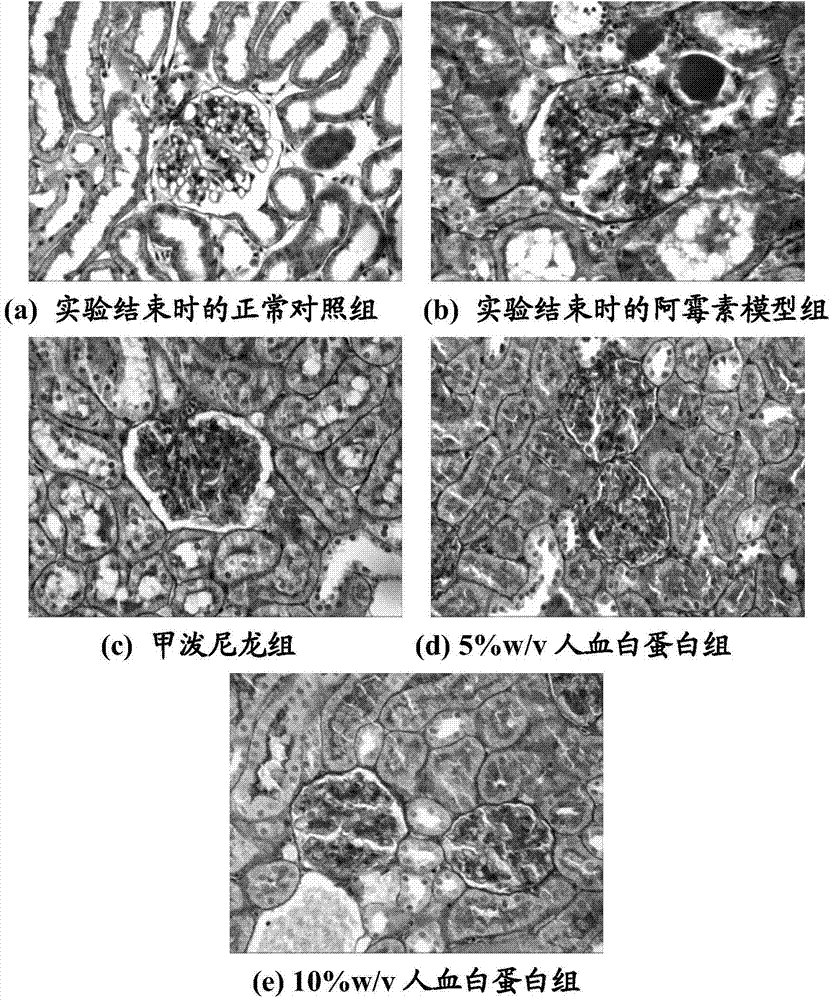
[0047] Example 3: Effect of the concentration of human serum albumin on proteinuria in NS rats
[0048] The 20% w / v human serum albumin for injection used in this test was from Jet Behring Pharmaceutical Co., Ltd., Germany, and other concentrations of human serum albumin were prepared from the above-mentioned 20% w / v human serum albumin for injection.
[0049] The preparation method of 2.5% w / v human serum albumin: take 3.75 mL of 20% w / v human serum albumin for injection, add 26.25 mL of sterile pyrogen-free normal saline, and mix well.
[0050] Preparation method of 5% w / v human serum albumin: Take 7.5 mL of 20% w / v human serum albumin for injection, add 22.5 mL of sterile pyrogen-free normal saline, and mix well.
[0051] The preparation method of 10% w / v human serum albumin: take 15 mL of 20% w / v human serum albumin for injection, add 15 mL of sterile pyrogen-free normal saline, and mix well.
[0052] The preparation method of 15% w / v human serum albumin: take 22.5 mL of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com