Pyrrole-containing triphenylamine conjugated schiff base compound, and preparation method and application thereof
A technology of pyrrolyltriphenylamine and Schiff base, applied in chemical instruments and methods, organic chemistry, color-changing fluorescent materials, etc., can solve the problems of high synthesis cost and complex synthesis method, and achieve the effect of high degree of conjugation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
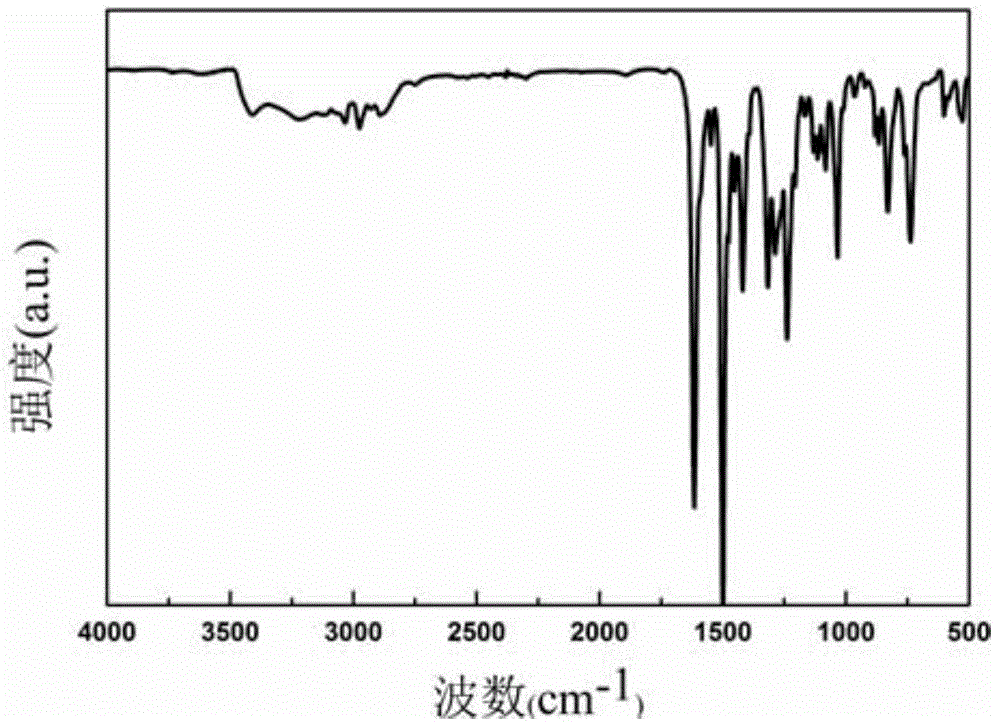
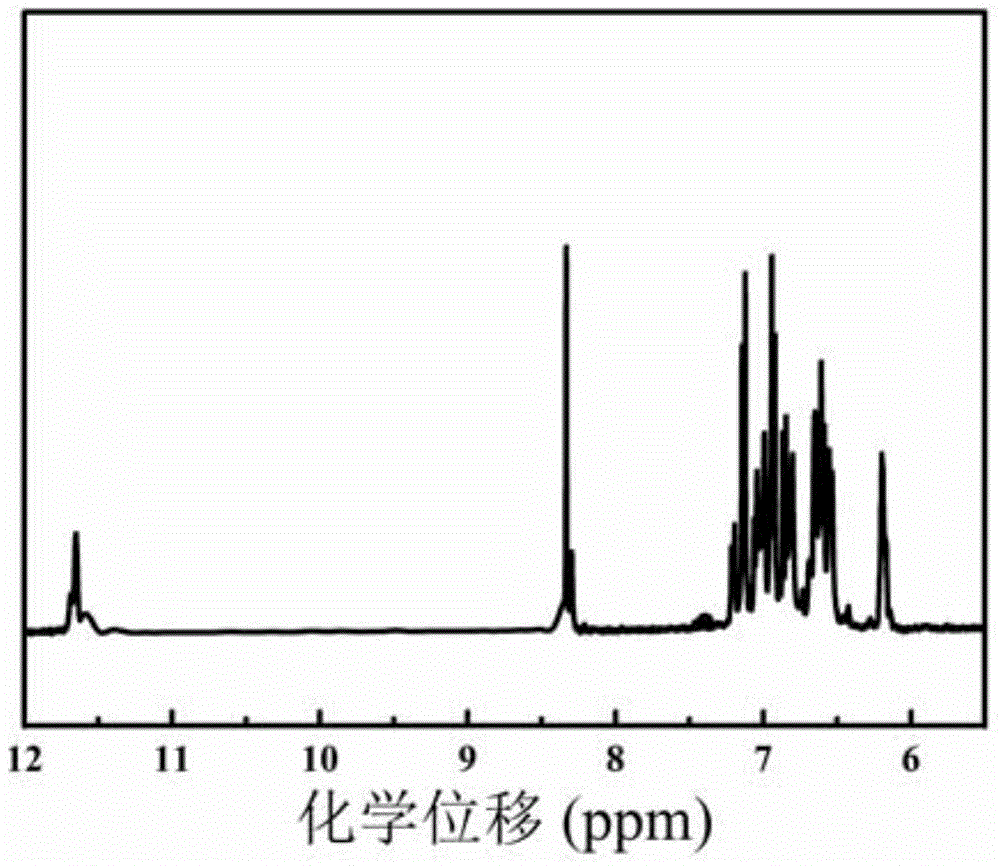
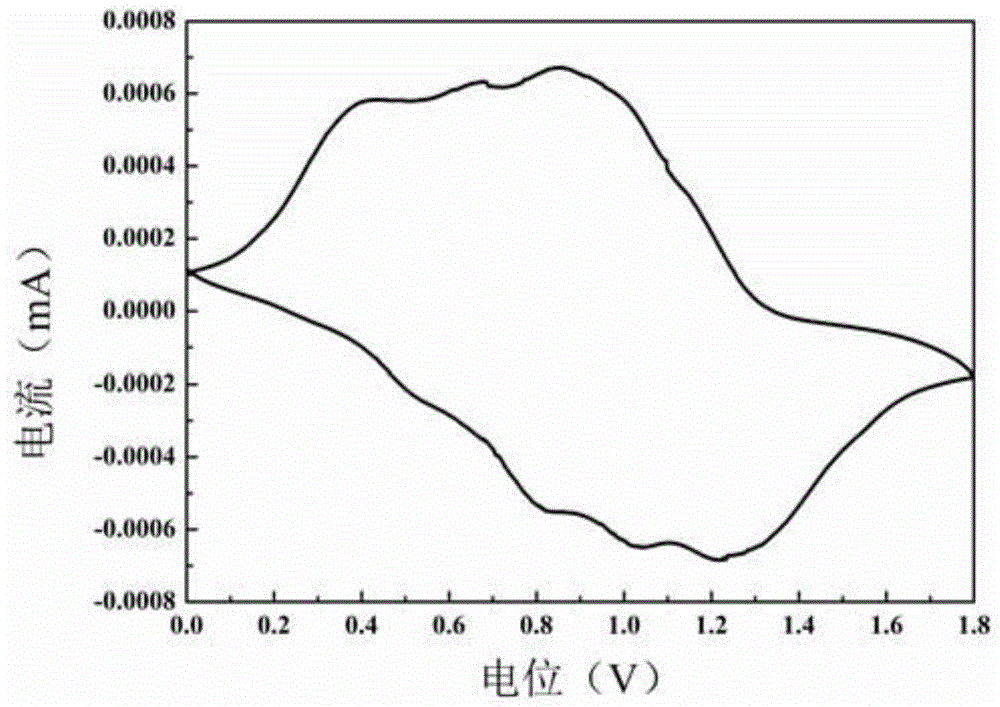
Image
Examples
specific Embodiment approach 1
[0042] Specific implementation mode 1: This implementation mode is a kind of pyrrolyltriphenylamine-containing conjugated Schiff base compound whose structural formula is as follows:
[0043] where the R 1 For H, R 2 for H; R 1 for when R 2 for H, -CH 3 or -OCH 2 CH 3 .
[0044] The beneficial effect of this implementation mode:
[0045]1. The Schiff base compound of the present embodiment changes the shortcomings of narrow application range and single function of Schiff base in the past, and provides a multifunctional Schiff base; a -C=N- double bond structure is formed in the molecule, and the double bond connects benzene Ring and pyrrole, forming a large conjugated structure, so that the electron cloud can be transported in a very long molecular chain, prone to light absorption, resulting in a color change after protonation in acid or oxidation with an applied voltage, and this change is reversible , accompanied by a reversible color change;
[0046] 2. In th...
specific Embodiment approach 2
[0047] Specific embodiment two: This embodiment is a preparation method of a conjugated Schiff base compound containing pyrrolyl triphenylamine, which is prepared according to the following method:
[0048] 1. Mix the triphenylamine derivative and absolute ethanol, and then magnetically stir for 10 minutes to 15 minutes at a temperature of 79°C to 80°C and a stirring speed of 200r / min to 300r / min to obtain an ethanol solution of the triphenylamine derivative;
[0049] The volume ratio of the mass of the triphenylamine derivative described in step 1 to absolute ethanol is 0.1g: (10mL~20mL);
[0050] 2. Under a nitrogen atmosphere, add pyrrole-2-carbaldehyde ethanol solution dropwise to triphenylamine derivative ethanol solution at a rate of 20 drops / min to 30 drops / min, and then react at a temperature of 79°C to 80°C 20h~24h, get the reactant;
[0051] The volume ratio of the mass of pyrrole-2-carbaldehyde in the pyrrole-2-carbaldehyde ethanol solution described in step 2 to a...
specific Embodiment approach 3
[0057] Specific embodiment three: the difference between this embodiment and specific embodiment two is: the triphenylamine derivative described in step one is Others are the same as in the second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com