Thiophene derivative based on 9-phenyl-carbazole as well as preparation and application of thiophene derivative
A technology of tributylstannyl dithiophene and its derivatives, applied in organic chemistry, chemical instruments and methods, color-changing fluorescent materials, etc., can solve the problem of high driving voltage, achieve increased conjugation degree, great application value, The effect of lowering the redox potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
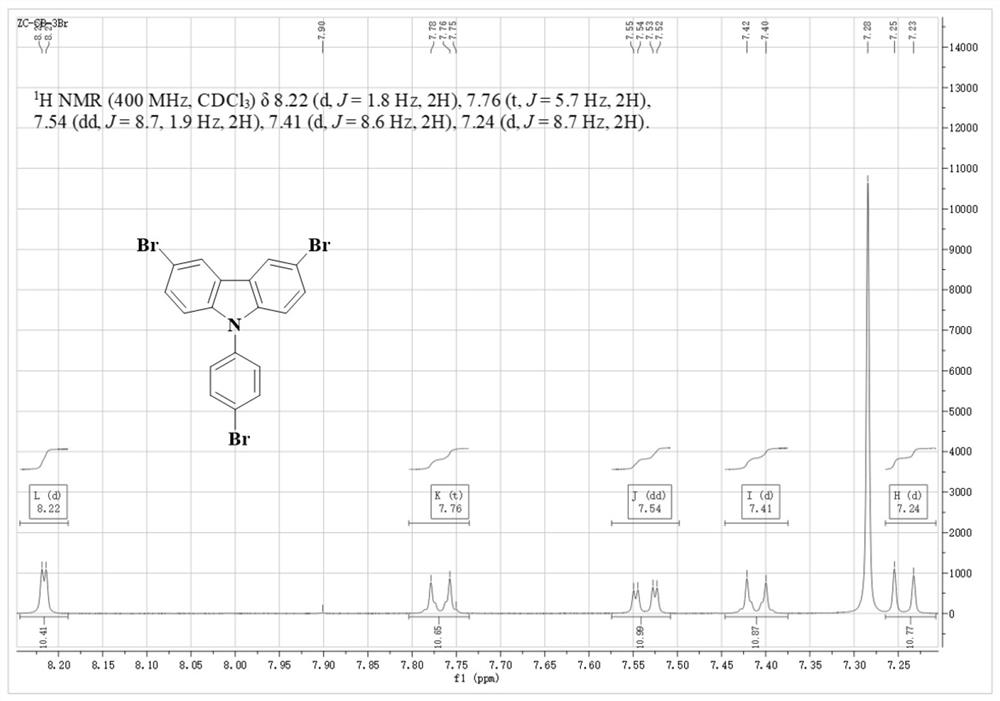
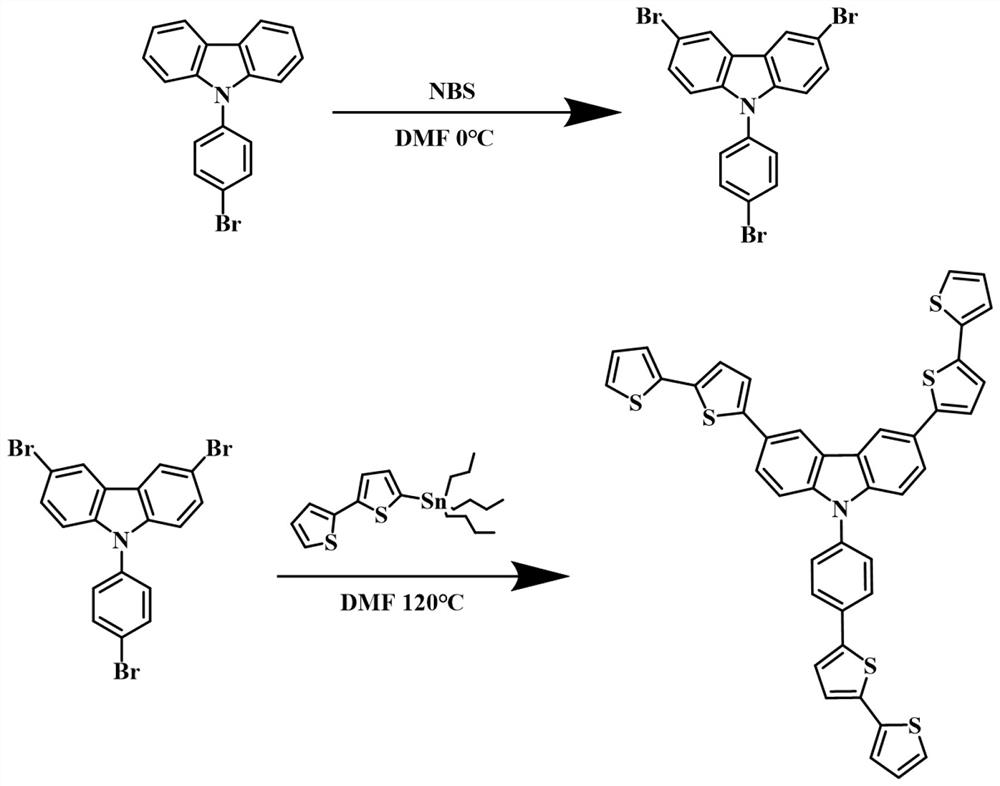
[0029] Example 1: Synthesis of TBTCB-3Br
[0030] Combine 9-(4-bromophenyl)-carbazole (0.5 g, 1.55 mmol), N-bromosuccinimide (1.65 g, 6.2 mmol) and N,N-dimethylformamide (30 mL) ) into a single-necked round-bottomed flask in turn, and stirred at 0 °C in an ice-water mixed bath for 8 hours. After the reaction was completed, pour the fully reacted solution into 200 mL of deionized water, and extract 5 times with 400 mL of dichloromethane. , until the solvent N, N-dimethylformamide was completely extracted, the extract was concentrated, dried with anhydrous sodium sulfate to remove water, mixed with crude silica gel (Qingdao Yumingyuan Silica Reagent Co., Ltd.), and then subjected to column chromatography Purification, using fine silica gel (Qingdao Yumingyuan Silica Reagent Co., Ltd.) as the stationary phase, dichloromethane and petroleum ether with a volume ratio of 1:4~5 as the mobile phase, collecting the eluent containing the target compound, and removing it by rotary evapor...
Embodiment 2
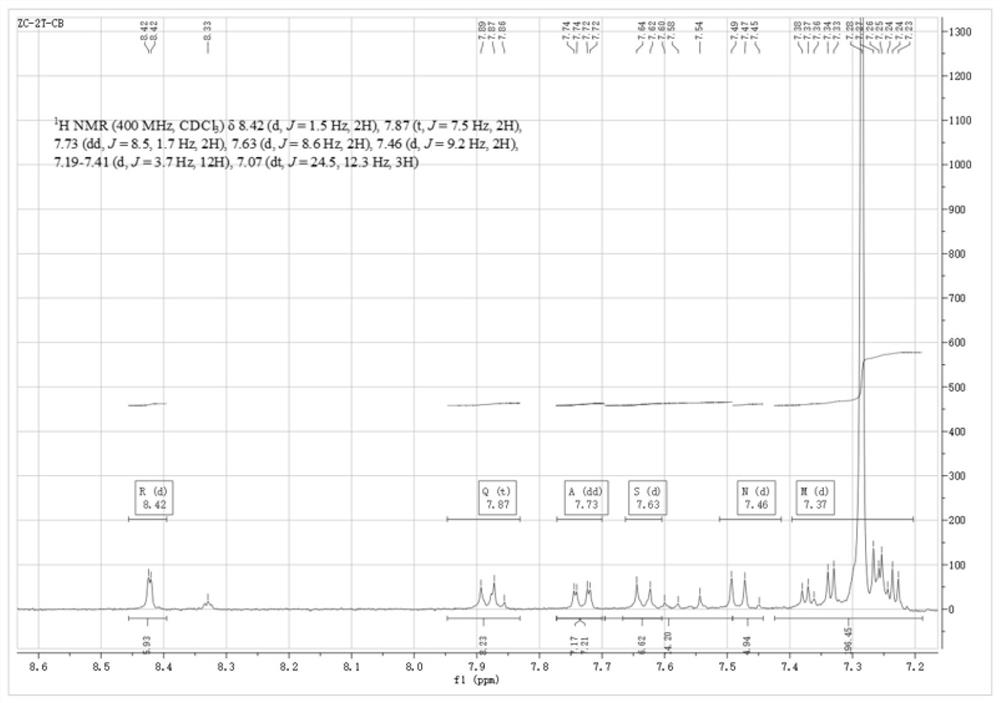
[0032] Example 2: Synthesis of Thiophene Derivatives of 9-Phenyl-carbazole (TBTCB-3T)
[0033] The TBTCB-3Br (0.8 g, 1.68 mmol) prepared in Example 1 and an appropriate amount of bis(triphenylphosphine) palladium dichloride were added to a 100 mL two-necked round-bottomed flask, and the mixture was heated under N 2 Under protection, 20 mL of N,N-dimethylformamide and 2-tributylstannyl dithiophene (6.86 g, 15.12 mmol) were added to the reaction flask in turn, stirred, and heated to reflux at 120 °C for 36 hours. After the reaction was completed, pour the completed solution into 200 mL of deionized water, and extract with 400 mL of dichloromethane for 5 times until the solvent N,N-dimethylformamide was completely extracted. After drying with sodium sulfate to remove water, the sample was mixed with crude silica gel, and then purified by column chromatography. Using fine silica gel as the stationary phase and dichloromethane and petroleum ether as the mobile phases, the eluate co...
Embodiment 3
[0035] Example 3: Preparation of polymer films of thiophene derivatives of 9-phenyl-carbazole
[0036] The thiophene derivatives of 9-phenyl-carbazole (TBTCB-3T) 7.35 mg (1 mmol / L) and the electrolyte tetrabutylammonium hexafluorophosphate 0.387 g (0.1 mol / L) were added to a 10 mL volumetric flask , dilute to volume with chromatographic grade dichloromethane, ultrasonicate for 3 min, and after the solid is completely dissolved, carry out electrochemical polymerization, and the polymerization curve is as follows Figure 4 shown. With ITO glass (0.9 4cm) is the working electrode, the platinum sheet is the counter electrode, and the Ag / AgCl is the reference electrode. The pTBTCB-3T polymer film was obtained by cyclic voltammetry polymerization. The polymerization voltage ranged from 0 to 1.5 V. 6 laps. Dedoping in a blank electrolyte solution (0.387 g tetrabutylammonium hexafluorophosphate, make up to 10 mL with chromatography grade dichloromethane) at a dedoping voltage of -0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com