Asymmetric organic small molecular photovoltaic material based on benzothiadiazole unit, preparation method and application thereof
A technology of benzothiadiazole and photovoltaic materials, applied in photovoltaic power generation, organic chemistry, electrical components, etc., can solve the problems of low photovoltaic devices, little research on asymmetric organic small molecule photovoltaic materials, lack of systematic research, etc., to achieve Effect of improving planarity and reducing HOMO energy level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
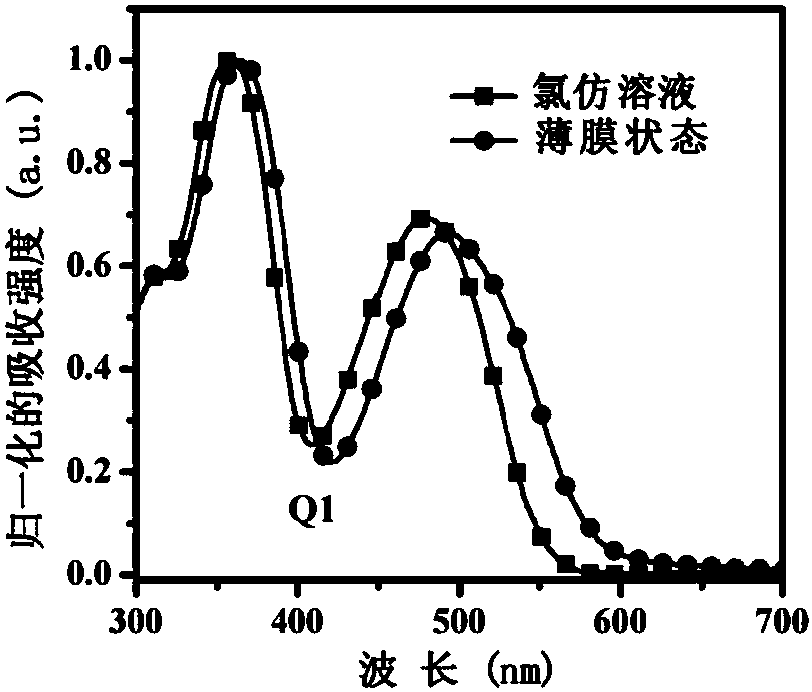
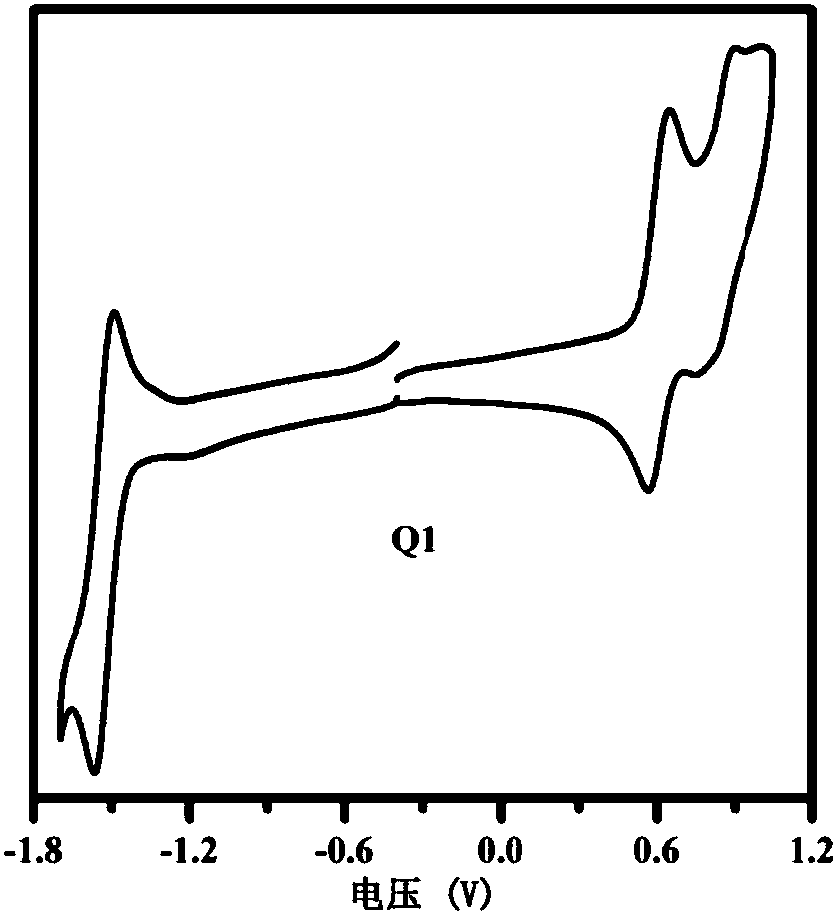
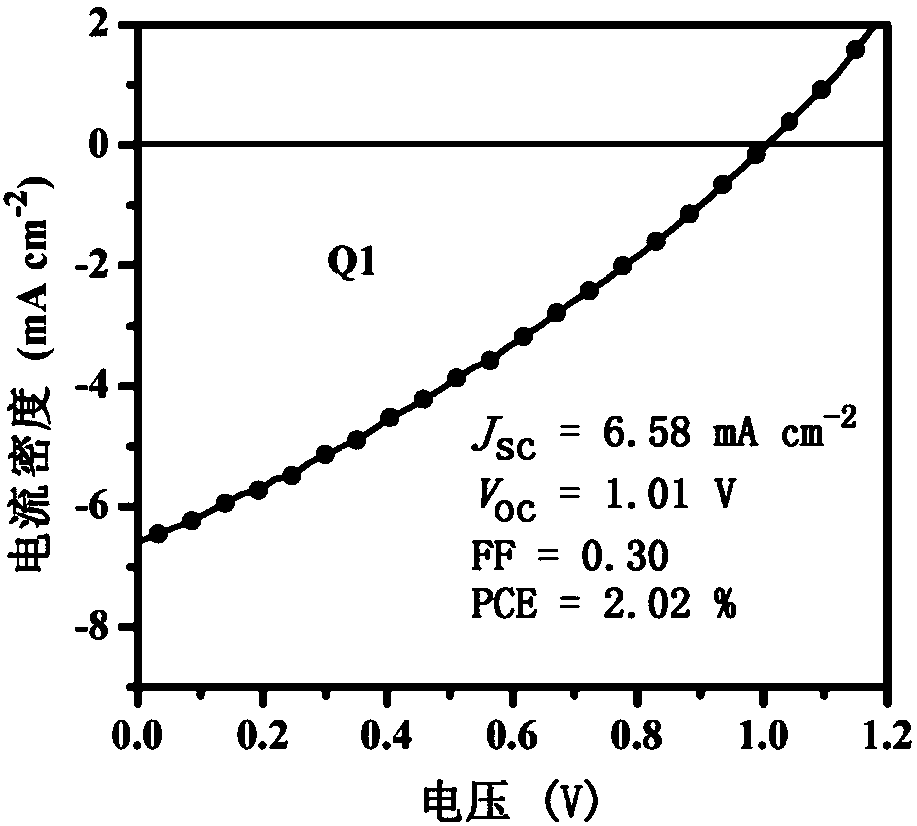
[0052] This example discloses the synthesis process of organic small molecule donor material Q1, the synthesis steps are as follows:
[0053] Under nitrogen protection, compound A (0.25mmol, 0.178g), compound D1 (0.30mmol, 0.134g), tetrakis (triphenylphosphine) palladium (0.025mmol, 29mg) and potassium carbonate (10mmol, 1.380 g) were placed Into a 50 mL three-necked flask, 10 mL of toluene, 5 mL of ethanol and 5 mL of water were sequentially added, and heated at reflux at 110° C. for 48 h. The reaction solution was cooled to room temperature, poured into 20 mL of water and extracted with dichloromethane (3×30 mL), the organic phases were combined, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure. Chloromethane was used as a developing solvent for column chromatography to obtain 0.143 g of a red solid with a yield of 60.0%.
[0054] The reaction formula of above-mentioned preparation compound Q1 is as follows:
[0055]
[0056] see ...
Embodiment 2
[0060] This embodiment discloses the synthesis process of organic small molecule donor material Q2, and the synthesis steps are as follows:
[0061] Under the protection of nitrogen, compound A (0.25mmol, 0.178g), compound D2 (0.30mmol, 0.142g), tetrakis (triphenylphosphine) palladium (0.025mmol, 29mg) and potassium carbonate (10mmol, 1.380 g) were placed Into a 50 mL three-necked flask, 10 mL of toluene, 5 mL of ethanol and 5 mL of water were sequentially added, and heated at reflux at 110° C. for 48 h. The reaction solution was cooled to room temperature, poured into 20 mL of water and extracted with dichloromethane (3×30 mL), the organic phases were combined, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure. Chloromethane was used as a developing solvent for column chromatography to obtain 0.159 g of a red solid with a yield of 65.0%.
[0062] The reaction formula of above-mentioned preparation compound Q2 is as follows:
[0063] ...
Embodiment 3
[0068] This example discloses the synthesis process of the organic small molecule donor material Q3, the synthesis steps are as follows:
[0069] Under nitrogen protection, compound A (0.25mmol, 0.178g), compound D3 (0.30mmol, 0.150g), tetrakis (triphenylphosphine) palladium (0.025mmol, 29mg) and potassium carbonate (10mmol, 1.380 g) were placed Into a 50 mL three-necked flask, 10 mL of toluene, 5 mL of ethanol and 5 mL of water were sequentially added, and heated at reflux at 110° C. for 48 h. The reaction solution was cooled to room temperature, poured into 20 mL of water and extracted with dichloromethane (3×30 mL), the organic phases were combined, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure. Chloromethane was used as a developing solvent for column chromatography to obtain 0.153 g of a red solid with a yield of 61.0%.
[0070] The reaction formula of above-mentioned preparation compound Q3 is as follows:
[0071]
[0072] s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com