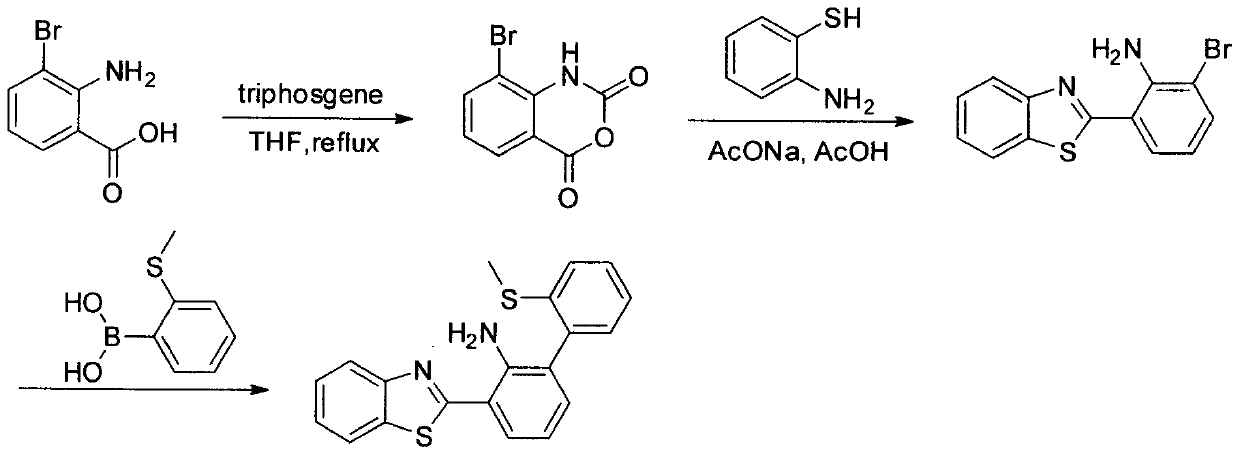
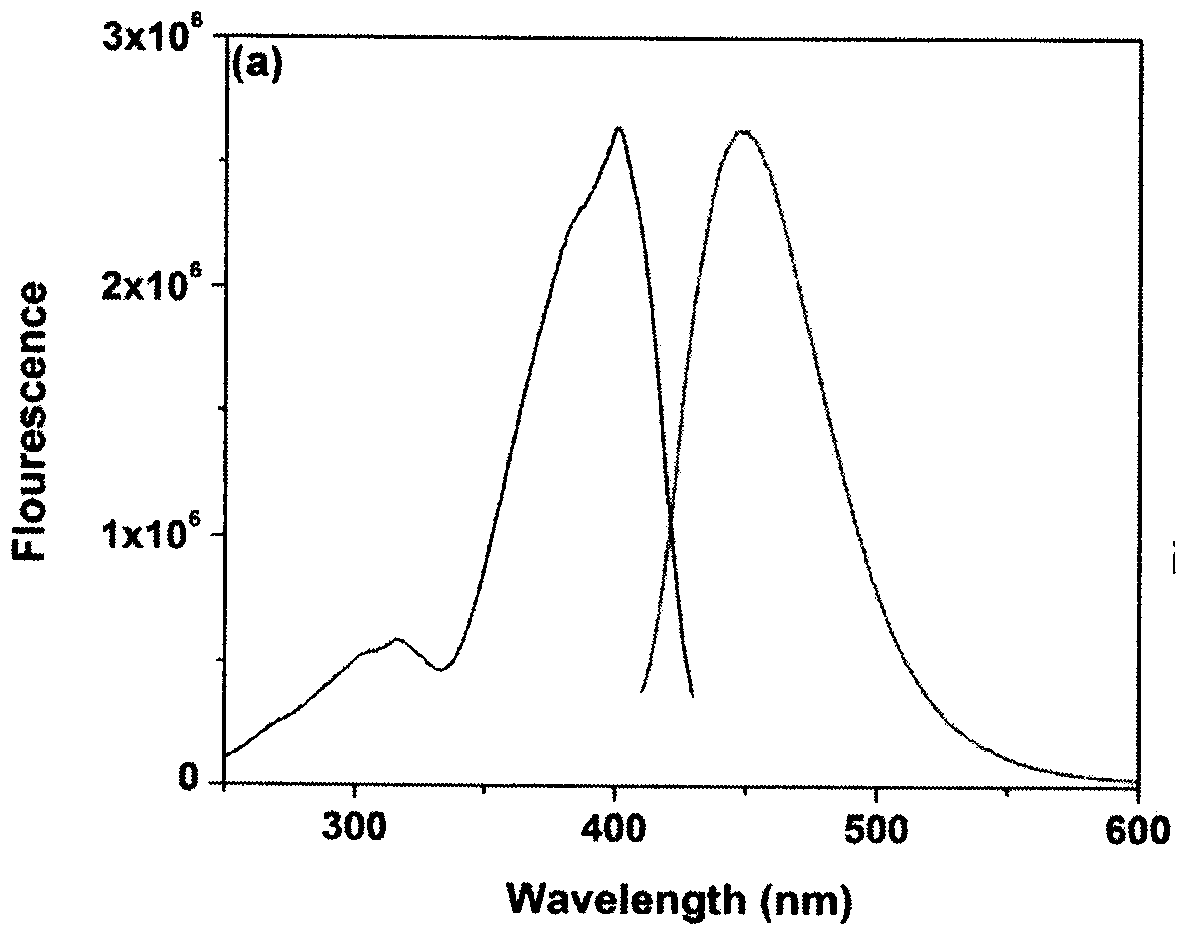
Benzothiazole compound and preparation method thereof, and application as probe for hypobromous acid
A technology of benzothiazole and compounds, applied in the application fields of benzothiazole compounds and their preparation, and hypobromous acid probes, achieving the effect of short response time and increased conjugation degree
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The synthetic method of 3-bromoisatoic anhydride:
[0040] Bis(trichloromethyl)carbonate (1.039g, 3.5mmol) was added to a tetrahydrofuran solution of 2-amino-3-bromobenzoic acid (2.150g, 10mmol), and the resulting mixed solution was refluxed and stirred for 4h, then quenched by adding 20mL of water extinguished, filtered with suction, washed the filter cake with methanol, and dried in vacuo to obtain 1.754 g of the target product with a yield of 73%. 1 H NMR (400MHz, DMSO) δ11.10(s, 1H), 8.02(dd, J=8.0, 1.4Hz, 1H), 7.95(dd, J=7.8, 1.4Hz, 1H), 7.19(t, J= 7.9Hz, 1H). 13 C NMR (101MHz, DMSO) δ159.59, 146.99, 140.59, 139.94, 139.93, 129.06, 124.99, 113.34, 108.39.
[0041] The synthetic method of 2-benzothiazolyl-6-bromoaniline:
[0042] Under argon protection conditions, 3-bromoisatoic anhydride (2.226g, 9.2mmol) and 2-aminothiophenol (1.540g, 12.3mmol) and sodium acetate (0.615g, 7.5mmol) were dissolved in 80mL of acetic acid , The resulting mixed solution was heated ...
Embodiment 2
[0046] Synthesis of probes for benzothiazole compounds:
[0047] In a 50 mL round bottom flask, 2-benzothiazolyl-6-bromoaniline (2.7 mmol), 2-methylthiophenylboronic acid (3.3 mmol) and 1,1'-bis(diphenylphosphino)ferrocene Palladium(II) dichloride dichloromethane compound (0.15 mmol) was dissolved in 5 mL of toluene solution. Under argon protection, 2 mL of K was added to the mixture 2 CO 3 Solution (3.5M), reacted at 70°C for 24h. After TLC tracking detects that the reaction is complete, it is cooled to room temperature. After filtration and concentration under reduced pressure, it was purified by column chromatography (eluent: ethyl acetate:petroleum ether=1:4) to obtain a light yellow solid-benzothiazole compound with a yield of 45%.
[0048] The synthetic method of 3-bromoisatoic anhydride and 2-benzothiazolyl-6-bromoaniline is the same as that of Example 1.
Embodiment 3
[0050] Synthesis of probes for benzothiazole compounds:
[0051] In a 50 mL round bottom flask, 2-benzothiazolyl-6-bromoaniline (3.3 mmol), 2-methylthiophenylboronic acid (3.9 mmol) and 1,1'-bis(diphenylphosphino)ferrocene Palladium(II) dichloride dichloromethane compound (0.15 mmol) was dissolved in 5 mL of toluene solution. Under argon protection, 2 mL of K was added to the mixture 2 CO 3 Solution (5M), reacted at 90°C for 24h. After TLC tracking detected that the reaction was complete, it was cooled to room temperature. After filtration and concentration under reduced pressure, it was purified by column chromatography (eluent: ethyl acetate:petroleum ether=1:6) to obtain a light yellow solid-benzothiazole compound with a yield of 46%.
[0052] The synthetic method of 3-bromoisatoic anhydride and 2-benzothiazolyl-6-bromoaniline is the same as that of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com