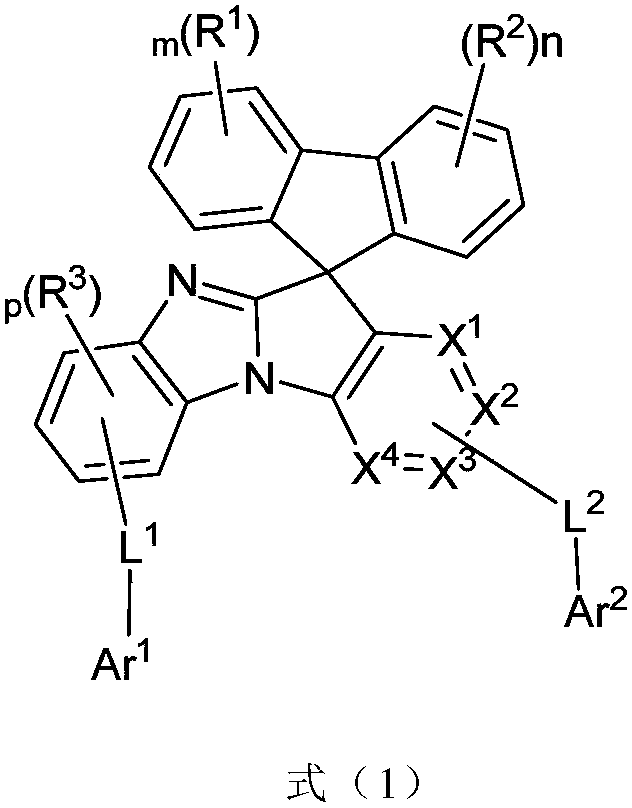
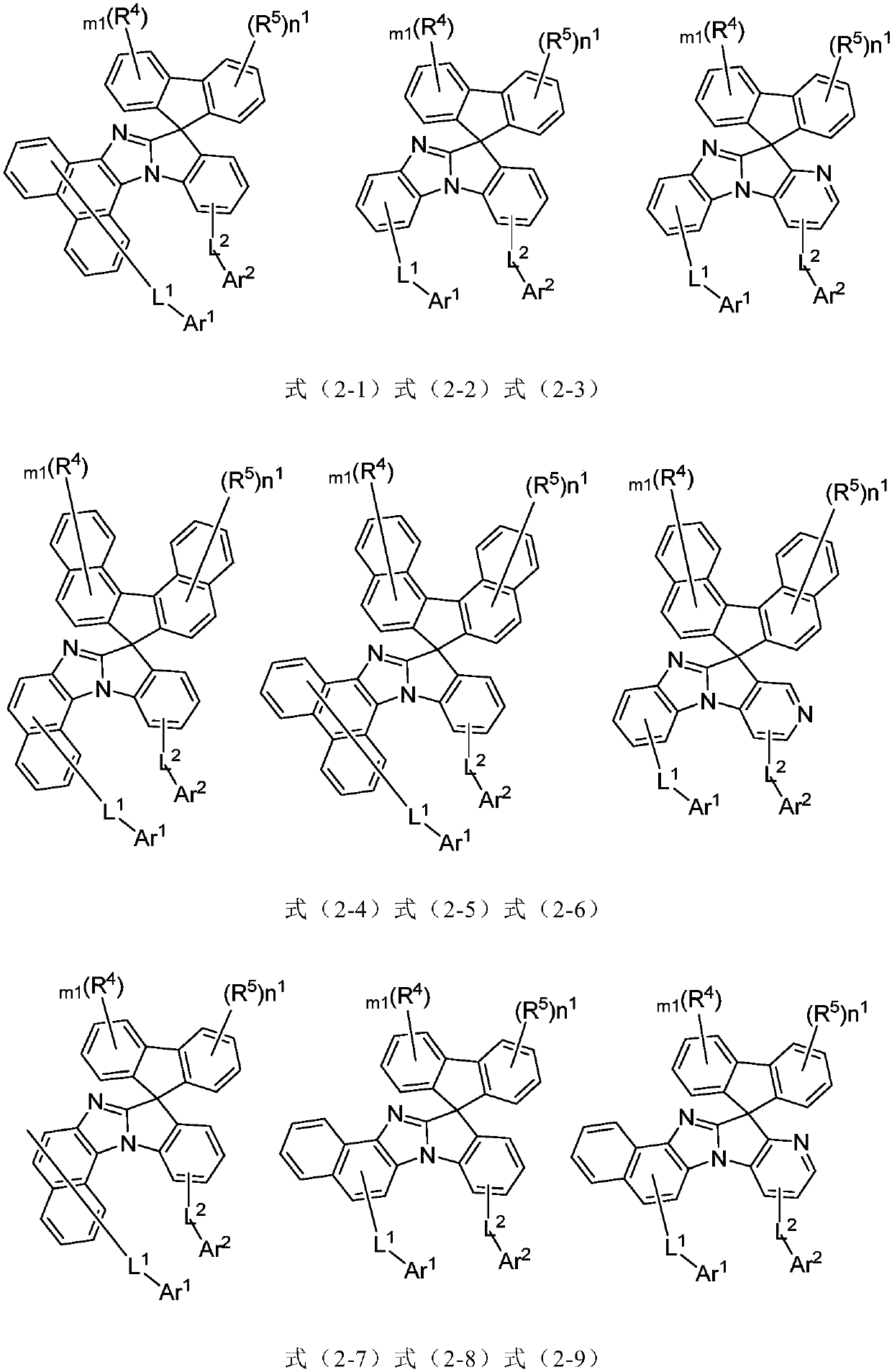
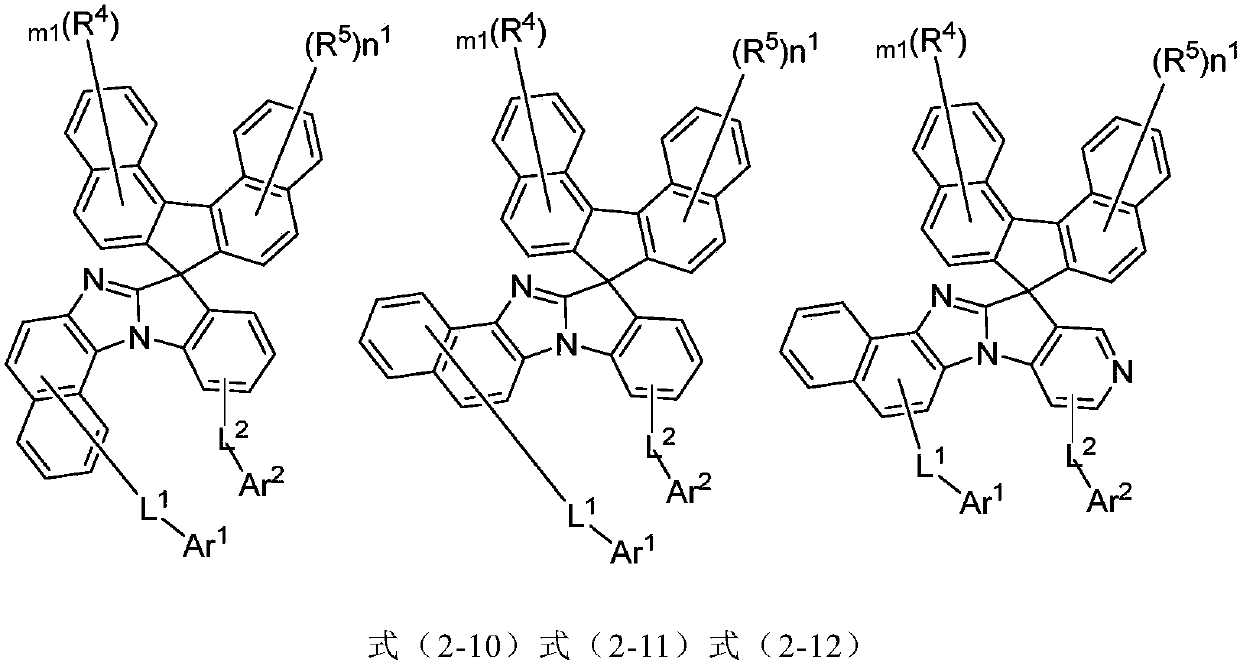
Imidazole substituted spirofluorene compound and applications thereof
A general formula compound, unsubstituted technology, applied in the field of imidazole-substituted spirofluorene organic compounds, organic electroluminescent devices, can solve the problems of weak electron transport ability and unbalanced carrier transport
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 2
[0068] Synthesis Example 2: Synthesis of M2:
Synthetic example 3
[0069] According to the synthesis of M1, the steps were the same, and 5-bromo-2-fluorobenzaldehyde was used instead of 2-fluoro-4-bromo-benzaldehyde to obtain 7.2 g of white solid, with a yield of 69%. The molecular ion mass determined by mass spectrometry is: 521.16 (calculated value: 521.19); theoretical element content (%) C 38 h 23 N 3 : C, 87.50; H, 4.44; N, 8.06. Measured element content (%): C, 87.47; H, 4.42; N, 8.06. The above analysis results indicated that the obtained product was the expected product. Synthesis Example 3: Synthesis of M12:
[0070] Take a dry 500mL two-necked bottle, add 8.68g (20mmol) M1-2, 7.2g (25mmol) 3-boric acid-9-phenylcarbazole, 4.14g (30mmol) anhydrous potassium carbonate, 230mg (0.2mmol) ) tetrakistriphenylphosphine palladium. After nitrogen replacement three times, 15 mL of distilled water and 250 mL of 1,4-dioxane were added, and heated to reflux for 12 h. After the solvent in the reaction system was distilled under reduced press...
Synthetic example 4
[0071] Synthesis Example 4: Synthesis of M46:
[0072] According to the synthesis of M12, the steps are the same, and 3-cyanophenylboronic acid is used instead of 3-boronic acid-9-phenylcarbazole for reaction to obtain 8.23 g of off-white solid with a yield of 90%. The molecular ion mass determined by mass spectrometry is: 457.14 (calculated value: 457.16); theoretical element content (%) C 33 h 19 N 3 : C, 86.63; H, 4.19; N, 9.18. Measured element content (%): C, 86.64; H, 4.17; N, 9.20. The above analysis results showed that the obtained product was the expected product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com