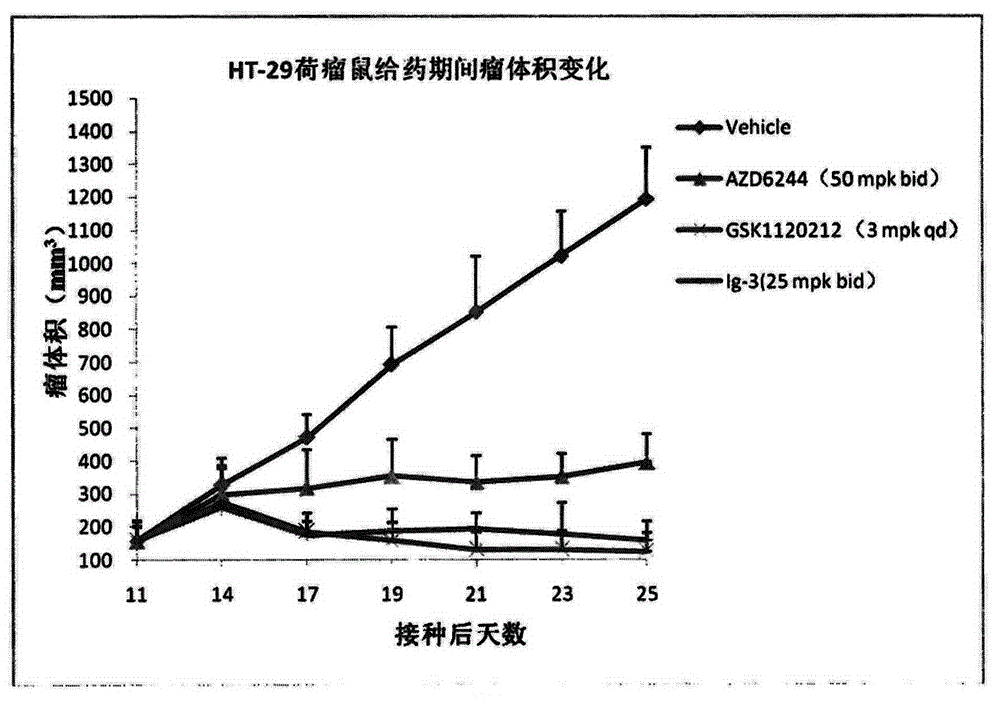
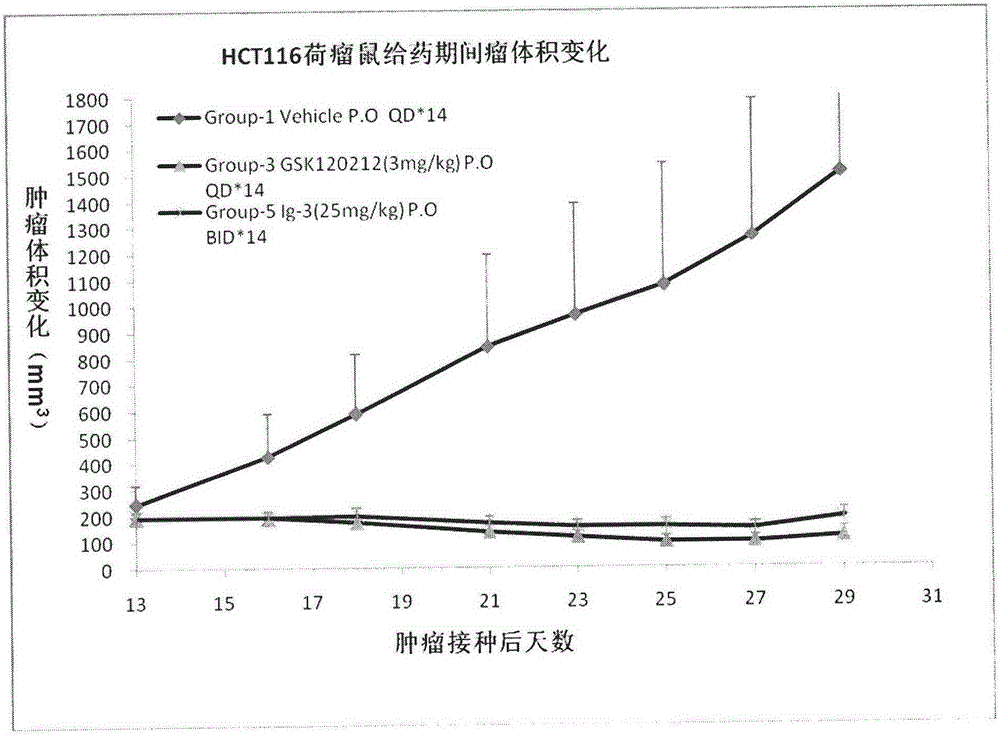
Isonicotinamide derivative, preparation method and applications thereof
A compound, selected technology, applied in the direction of drug combination, pharmaceutical formula, active ingredient of heterocyclic compounds, etc., to achieve effective cell proliferation and effective inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0143] Example 1: 3-[(3-tert-butoxycarbonylamino)phenylthio]-5-[(2-fluoro-4-iodophenyl)amino]isonicotinamide (Ie-1)
[0144]
[0145] Compound (d-1) was synthesized by referring to the preparation method of PCT patent WO2012 / 055953 intermediate 2.A. Compound (d-1) (0.35mmol), tert-butyl (3-mercaptophenyl)carbamate (0.455mmol), cesium carbonate (1.05mmol) were reacted at 60°C for 4 hours in DMF (7mL), added 20ml of water, and used Ethyl acetate was repeatedly extracted three times, and the organic phases were combined, washed twice with saturated brine, and dried over anhydrous sodium sulfate. Preparation, separation and purification to obtain 0.25 mmol of solid 3-[(3-tert-butoxycarbonylamino)phenylthio]-5-[(2-fluoro-4-iodophenyl)amino]isonicotinamide (Ie-1). The rate is 71.4%.
[0146] ESI (+) m / z: 581
Embodiment 2
[0147] Example 2: 3-(3-aminophenylthio)-5-[(2-fluoro-4-iodophenyl)amino]isonicotinamide (If-2)
[0148]
[0149] Compound (Ie-1) (0.5mmol) was dissolved in 20ml of ethyl acetate, 5ml of trifluoroacetic acid was added at 0°C, stirred overnight at room temperature, neutralized by adding 40ml of saturated sodium bicarbonate solution, the organic phase was separated, and the organic phase was anhydrous sulfuric acid Sodium drying, separation and purification to obtain solid 3-(3-aminophenylthio)-5-[(2-fluoro-4-iodophenyl)amino]isonicotinamide (If-2) 0.41mmol, yield 82 %.
[0150] ESI (+) m / z: 481
[0151] h 1 -NMR (deuterated DMSO): δ8.13(s, 1H), δ8.04(s, 1H), δ7.88(d, 2H), δ7.61(d, 1H), δ7.47(s, 1H), δ7.41(d, 1H), δ7.04(t, 1H), δ6.93(t, 1H), δ6.63(s, 1H), δ6.54(dd, 2H), δ5. 30(s, 2H).
Embodiment 3
[0152] Example 3: 3-[(3-acetylamino)phenylthio]-5-[(2-fluoro-4-iodophenyl)amino]isonicotinamide (Ig-3)
[0153]
[0154] Compound (If-2) (0.26mmol) was dissolved in a mixed solvent of 3ml pyridine and 3ml dichloromethane, and acetyl chloride (0.52mmol) was added under ice-cooling. Stir at room temperature for 5 hours, add 10 ml of water and 10 ml of dichloromethane, separate the organic phase, and dry the organic phase over anhydrous sodium sulfate. Preparation, separation and purification to obtain solid 3-[(3-acetylamino)phenylthio]-5-[(2-fluoro-4-iodophenyl)amino]isonicotinamide (Ig-3) 0.21mmol, yield 81 %.
[0155] ESI (+) m / z: 523
[0156] h 1 -NMR (deuterated DMSO): δ10.05(s, 1H), δ8.18(s, 1H), δ8.07(s, 1H), δ7.90-7.93(d, 2H), δ7.68( s, 1H), δ7.59(d, 1H), δ7.51(m, 2H), δ7.42(d, 1H), δ7.32(t, 1H), δ7.05(d, 1H), δ6.96 (t, 1H), δ7.05 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com