New method of synthesizing 2,4-di-substituted benzothiazole
A technology of benzothiazole and disubstitution, applied in the field of synthesizing 2,4-disubstituted benzothiazole, which can solve the problems of low yield, cumbersome process, and low yield in the ring-closing step
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
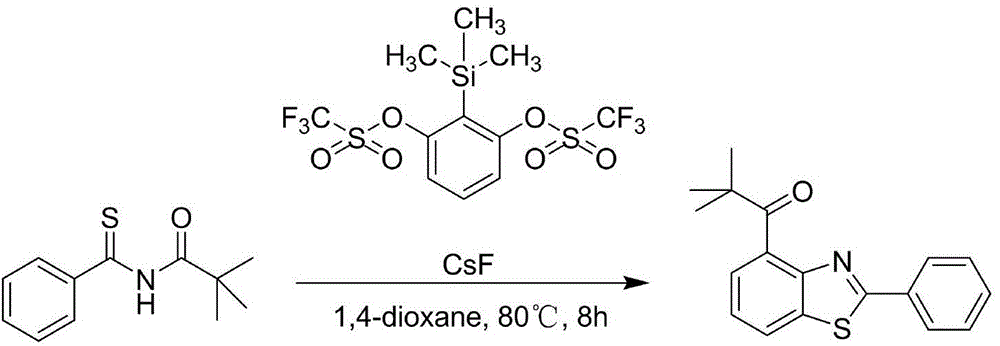
[0007] Below in conjunction with concrete reaction, further illustrate the present invention. Take R' as a trimethylacetyl group and R as a phenyl group as an example.
[0008] Trimethylacetyl-protected thioamide substrate (88.6 mg, 0.4 mmol, 1.0 equiv.), CsF (304 mg, 2 mmol, 5.0 equiv.) was dissolved in 1,4-dioxane (30 mL) reaction solvent , TPBT (268.0 mg, 0.6 mmol, 1.5 equiv.) in 1,4-dioxane (10 mL) was slowly added to the reaction solution at 80° C. over 8 hours, mixed uniformly and reacted.
[0009] Post-processing: After the reaction, the solvent was removed by a rotary evaporator, and the product 2-phenyl-4-trimethylacetylbenzothiazole was obtained by separation and purification on a silica gel column with a yield of 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com