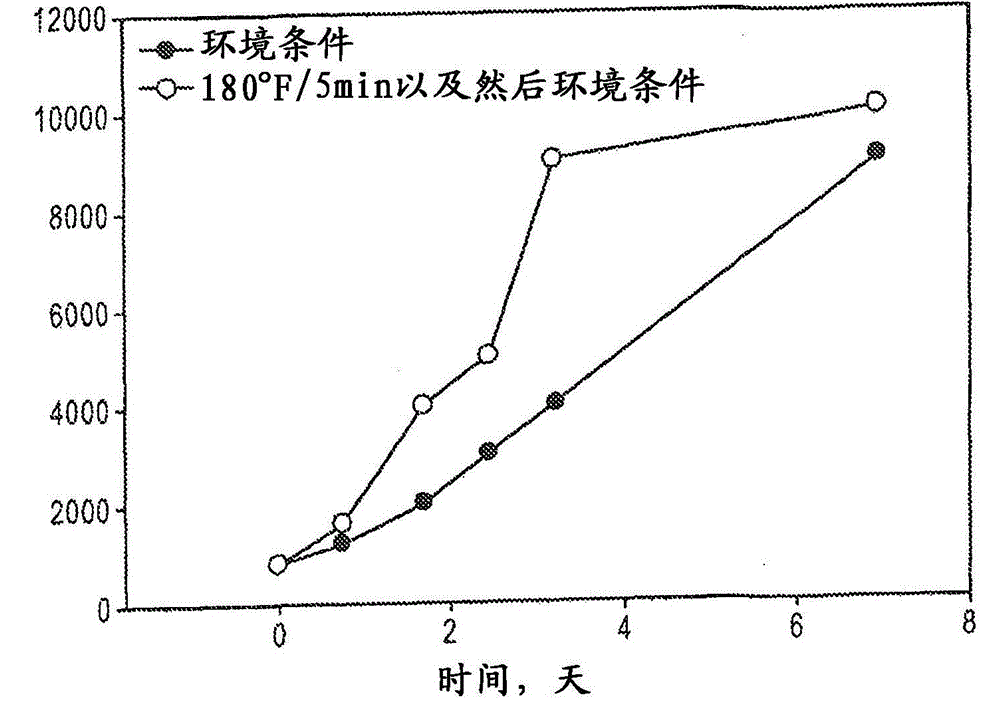
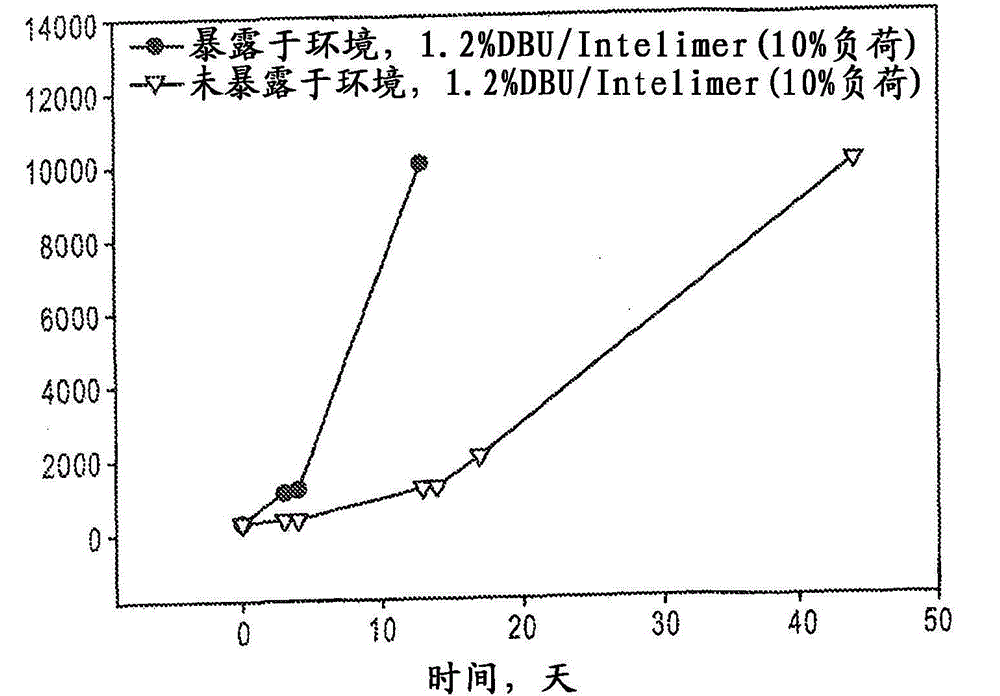
Controlled-release amine-catalyzed, sulfur-containing polymer and epoxy compositions
A composition and polymer technology, applied in the fields of compounds of group 4/14 elements of the periodic table, chemical instruments and methods, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0196] Trialkylsilane-terminated polysulfides and their preparation are disclosed, for example, in US Patent No. 4,902,736. In certain embodiments, the polysulfide comprises a thiol-terminated polysulfide such as under the name Commercially available from Akzo Nobel and under the name Those commercially available from Toray.
[0197] The terminal thiol groups of the thiol-terminated polysulfide can be converted to trialkylsilane groups by, for example, reacting the thiol-terminated polysulfide with a halosilane such as that of formula (9) in a base The reaction is carried out in the presence of a neutral catalyst, including an amine catalyst such as triethylamine. Examples of suitable halosilanes include trimethylchlorosilane, trimethylbromosilane, trimethyliodosilane, dimethylphenylchlorosilane, and chloromethyldimethylsilyl chloride. Examples of suitable halosilanes further include triethylchlorosilane, triethylbromosilane, triethyliodosilane, diethylphenylchlorosilane,...
Embodiment 1
[0260] Controlled Release Catalyst Preparation
[0261] 9.37g of 13-6 (from Air Products and Chemicals, Allentown, PA) and 0.63 g of 1,8-diazabicyclo-5,4,0-undecene-7 (DBU) were blended at 80°C for 30 minutes. The mixture was rapidly cooled to room temperature and then ground to a powder with an average particle size of 20-50 microns.
Embodiment 2
[0263] Controlled Release Catalyst Preparation
[0264] 9.00g of 13-6 (from Air Products and Chemicals, Allentown, PA) and 1.00 g of 1,8-diazabicyclo-5,4,0-undecene-7 (DBU) were blended at 80°C for 30 minutes. The mixture was rapidly cooled to room temperature and then ground into a powder.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com