Method for detecting toxigenic pasteurellamultocida toxin formaldehyde inactivated efficacy by using Vero cells (African Green Monkey Kidney cells)
A technology for the inactivation of Pasteurella and formaldehyde, which is applied in the direction of biochemical equipment and methods, and the determination/inspection of microorganisms. problem, to achieve the effect of ensuring safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0013] Preparation of bacterial solution: inoculate the toxin-producing Pasteurella multocida into the brain-heart infusion medium, place it in a constant temperature shaking incubator at 36-38°C, and cultivate it at 200r / min for 12-18 hours, and harvest the bacterial solution.
[0014] Inactivation of the bacterial liquid: add formaldehyde to the toxigenic Pasteurella multocida bacterial liquid respectively according to the final concentration (v / v) of 0.2%, 0.25% and 0.3%, put in a constant temperature shaking incubator at 36~38°C, and set the temperature at 200r / min for 36, 48 and 60 hours respectively. Take 10ml of inactivated bacteria solution at each time point for use.
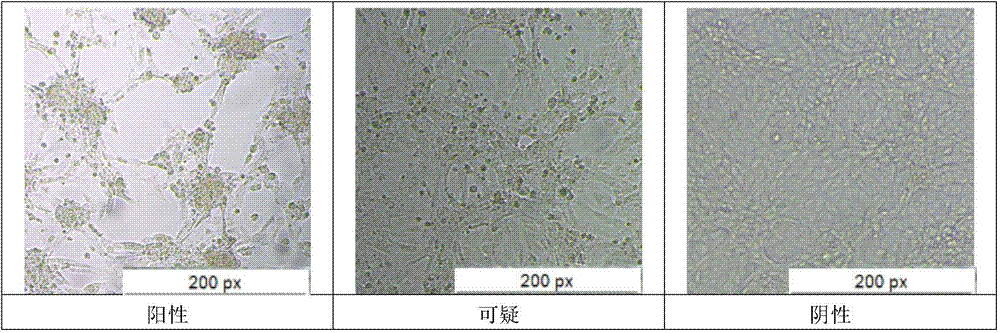
[0015] Guinea pig skin necrosis detection Take 0.1ml bacterial solution for each concentration and each time period, inject guinea pigs intradermally on the back, observe for 72 hours after injection, and measure the size of the skin necrosis area at the injection point. A necrotic area with a diamete...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com