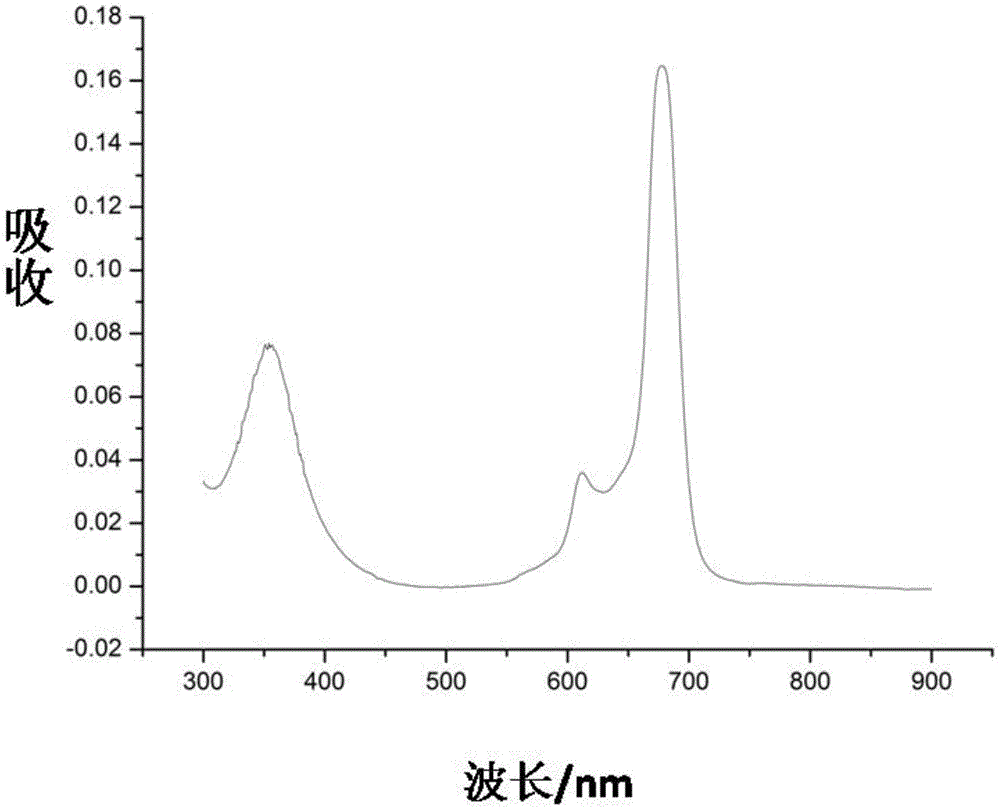
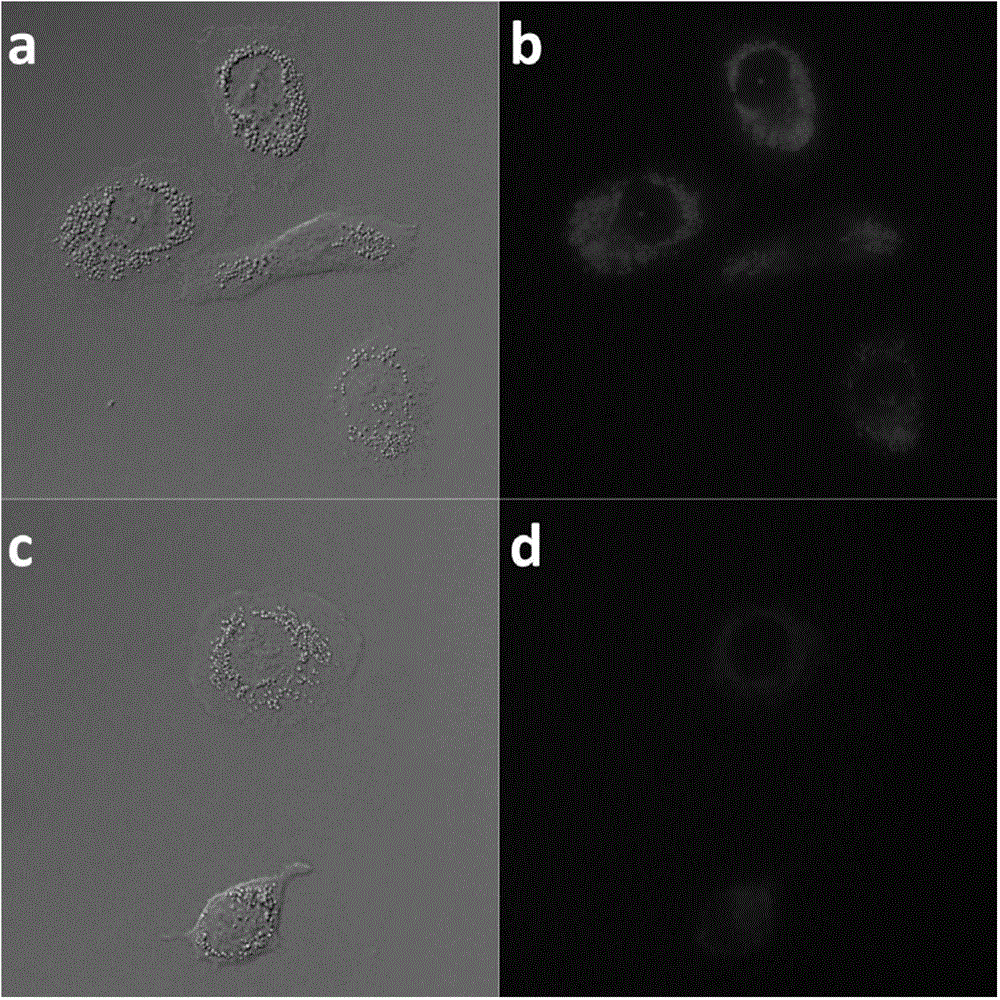
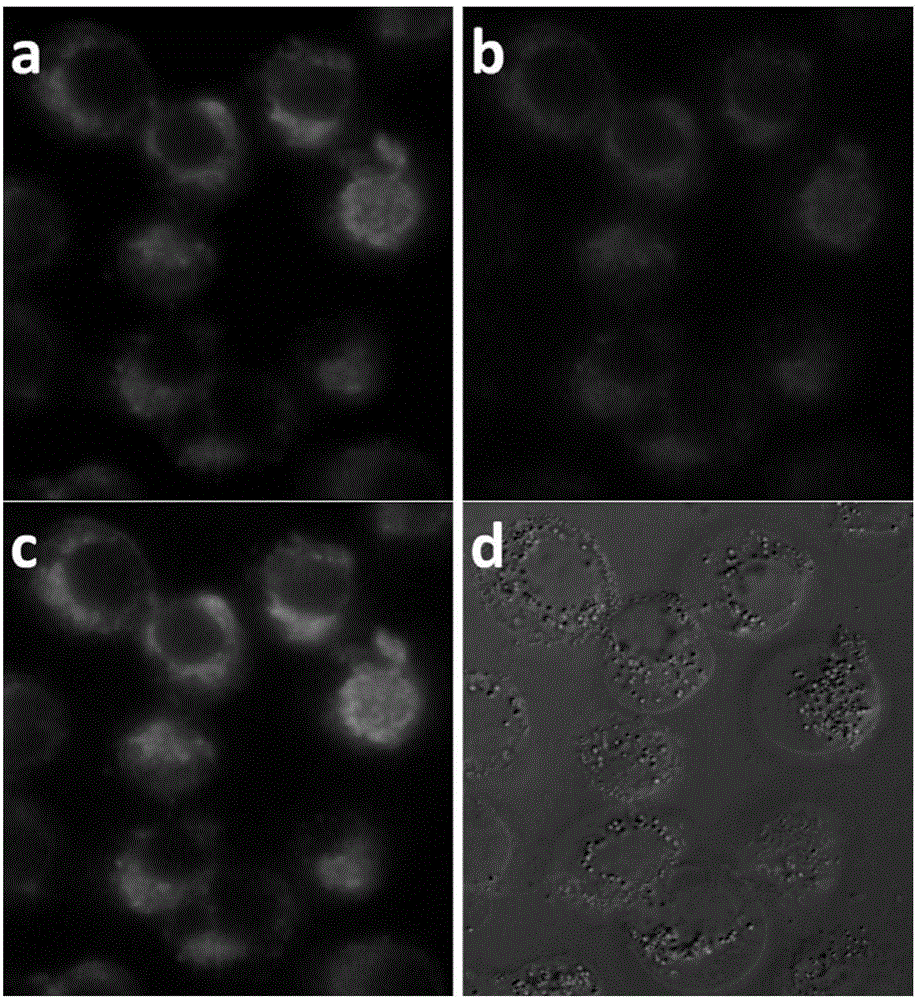
Phthalocyanine compound, preparation method and application as single/two-photon fluorescent probe in cancer targeting and mitochondria labeling
A compound and phthalocyanine technology, applied in the field of phthalocyanine-based compounds and their preparation, can solve the problems of non-phototoxicity, limited application of phthalocyanine, and destructiveness of biological tissues of research objects, and achieves good affinity, large absorption cross-section, and good application. effect of value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] The preparation of the phthalocyanine base compound shown in embodiment 1, formula (II)
[0061] (1) Preparation of ethyl 4-[3,4-dicyanophenoxy]butanoate (a)
[0062]
[0063] Weigh 0.55g (3.8mmol) 4-hydroxyphthalonitrile and 1.3g (6.9mmol) ethyl 4-bromo-n-butyrate, and 1.6g K 2 CO 3 Dissolve in DMF (10mL), stir at room temperature for 24h, then drain the DMF under reduced pressure, dissolve the residue in chloroform and wash with water three times, collect the organic layer and remove the organic solvent chloroform under reduced pressure, the crude product is separated by silica gel column chromatography to obtain White solid, yield 83.6%.
[0064] 1 H NMR (300MHz, CDCl 3 , 25℃, TMS): δ=7.72-7.70(d, J=8.7Hz, 1H, ArH), 7.27-7.17(m, 2H, ArH), 4.20-4.10(m, 4H, CH 2 ), 2.52(t, J=7.2Hz, 2H, CH 2 ),2.20-2.12(m,2H,CH 2 ), 1.27(t, J=7.2Hz, 3H, CH 3 );
[0065] (2) Preparation of three [(4-tert-butyl)phenoxy]-one[(4-n-butyrate)phenoxy]zinc phthalocyanine (c)
[006...
Embodiment 2
[0077] Embodiment 2, center ion is Ni 2+ Preparation of phthalocyanine-based compounds
[0078] The detailed steps are the same as in Example 1, except that the metal salt is changed to nickel acetate in the step (2).
Embodiment 3
[0079] Embodiment 3, center ion is Mg 2+ Preparation of phthalocyanine-based compounds
[0080] The detailed steps are the same as in Example 1, except that the metal salt is changed to magnesium chloride in the step (2).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com