A kind of manganese vanadate nanometer material and its synthesis method, application
A technology to synthesize manganese vanadate nanometer and manganese vanadate nanometer, applied in the field of inorganic non-metallic materials, can solve the problems of high energy consumption, large size, long cycle, etc., and achieve the effect of controllable conditions, uniform size and pure product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 4 mmol of ammonium metavanadate (NH 4 VO 3 , analytically pure) was dissolved in 8 mL of distilled water, and 2 mmol of manganese chloride (MnCl 2 · 4H 2 O, analytical grade) was dissolved in 4 mL of distilled water, and the NH 4 VO 3 The solution was added dropwise to MnCl 2 solution, make a reaction system, stir for 10 min, with 1mol / L HNO 3 (Analytical pure) solution Adjust the pH value of the reaction system to 2, continue to stir for 10 min, transfer it to a 20 mL hydrothermal reaction kettle, seal it, and place it in a thermostat for 3 hours at 120°C for hydrothermal reaction. After the reaction is completed, Cool to room temperature, centrifuge to obtain a precipitate, wash the precipitate five times with deionized water and absolute ethanol, and finally centrifuge and place the obtained precipitate in an oven, and dry it in vacuum at 60 °C for 16 h to obtain manganese vanadate MnV 12 o 31 10H 2 O nanomaterials.
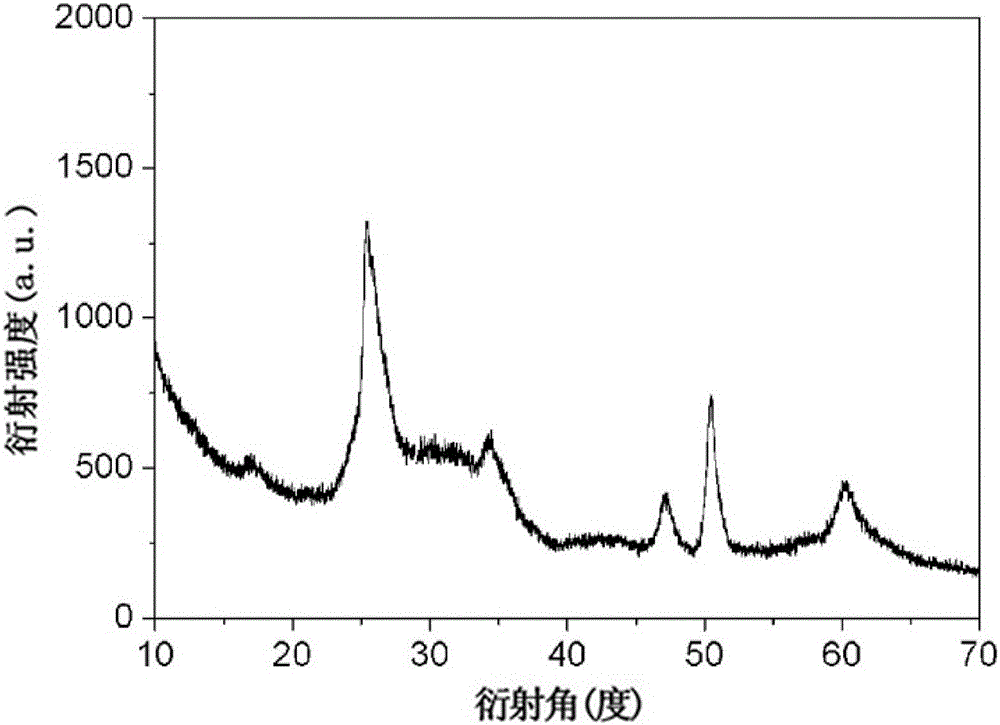
[0037] Carry out phase analysis with X-r...
Embodiment 2
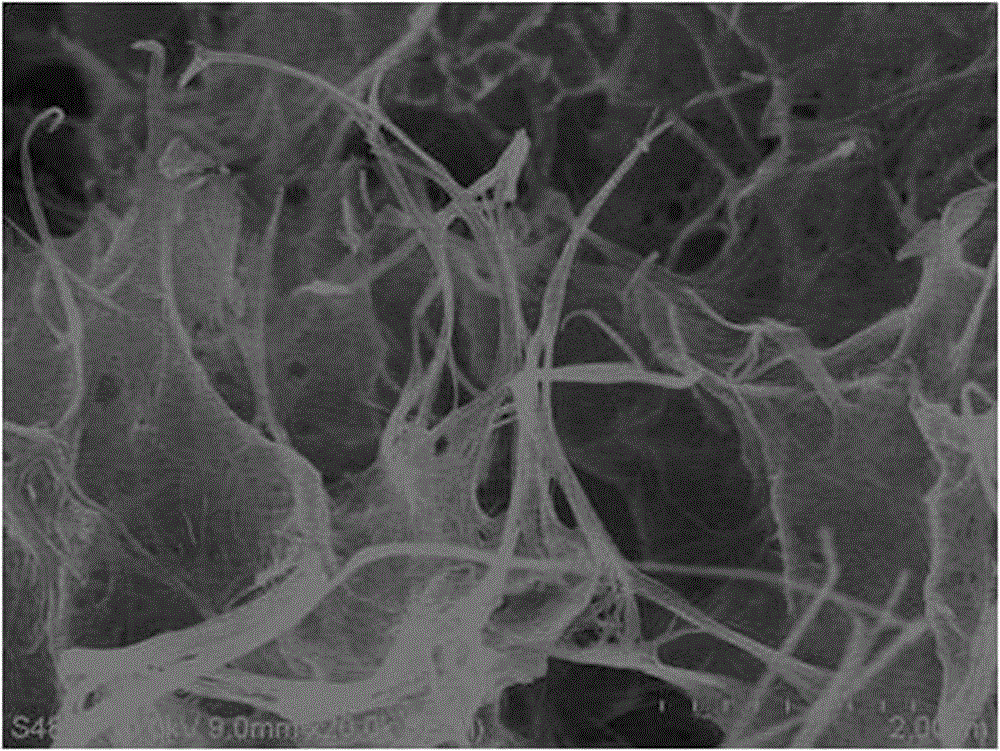
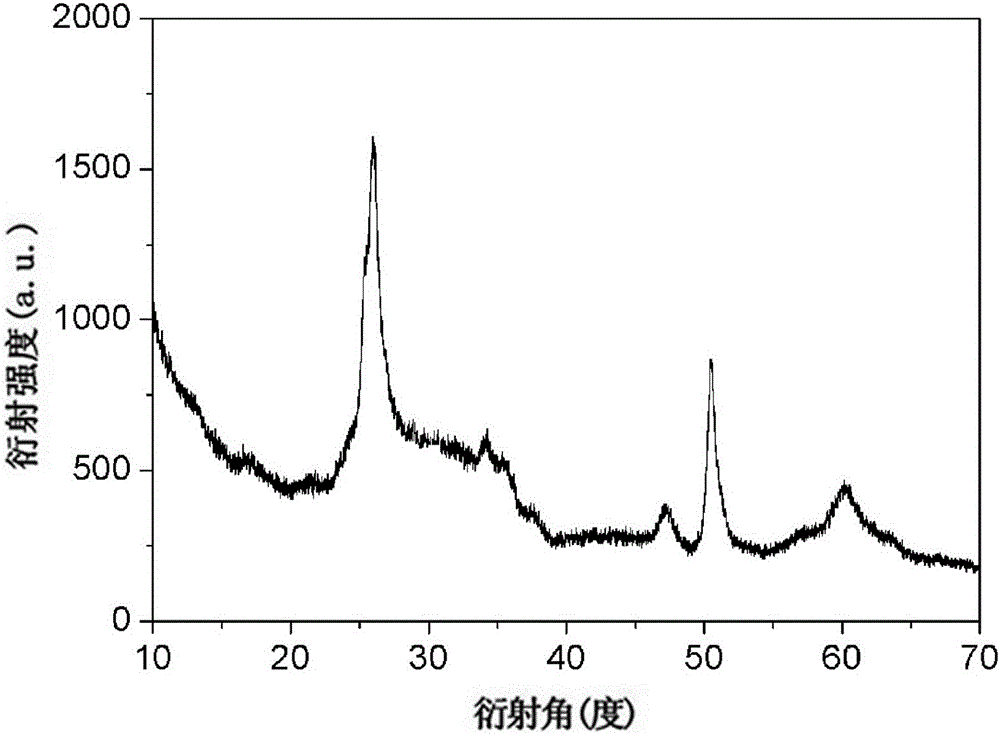
[0039] 0.2mmol of NH 4 VO 3 Dissolved in 8 mL of distilled water, 0.2 mmol of MnCl 2 · 4H 2 O was dissolved in 4 mL of distilled water, and the NH 4 VO 3 The solution was added dropwise to MnCl 2 solution, stirred for 10 min, with 1mol / L HNO 3 The solution adjusts the pH value of the reaction system to 2 and continues to stir for 15 min, then transfers it into a 20 mL hydrothermal reaction kettle, seals it and places it in a constant temperature box for 1 hour of hydrothermal reaction at 160 °C, and cools to room temperature after the reaction is completed , centrifuged to obtain a precipitate, washed with deionized water and absolute ethanol for 3 times, and dried in an oven at 60 °C for 16 h under vacuum to obtain MnV 12 o 31 10H 2 O material. The results of phase and morphology analysis are as follows: image 3 , Figure 4 As shown, it can be seen that the characteristic peak position and intensity of the product are related to the MnV 12 o 31 10H 2 O standard...
Embodiment 3
[0041] 8mmol of NH 4 VO 3 Dissolved in 8 mL of distilled water, 4 mmol of MnCl 2 · 4H 2 O was dissolved in 4 mL distilled water, and the NH 4 VO 3 The solution was added dropwise to MnCl 2 solution, stirred for 10 min, with 1mol / L HNO 3 The solution adjusts the pH value of the reaction system to 1 and continues to stir for 5 min, then transfers it into a 20 mL hydrothermal reaction kettle, seals it and places it in a constant temperature box for 6 hours of hydrothermal reaction at 110 °C, and cools to room temperature after the reaction , centrifuged to obtain a precipitate, washed five times with deionized water and absolute ethanol, and placed the obtained precipitate in an oven at 60 °C for 16 h in vacuum to obtain MnV 12 o 31 10H 2 O material. The results of phase and morphology analysis are as follows: Figure 5 , Image 6 As shown, the XRD spectrum results show that the intensity and position of the product diffraction peaks are related to the MnV 12 o 31 10...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com