Preparation method and application of multi-target complex antigen-loaded CD8+ cytotoxic T lymphocytes
A technology of lymphocytes and compound antigens, applied in the fields of biology and medicine, can solve the problem of tumor heterogeneity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1 Construction of lentiviral LentihGM-CSF vector
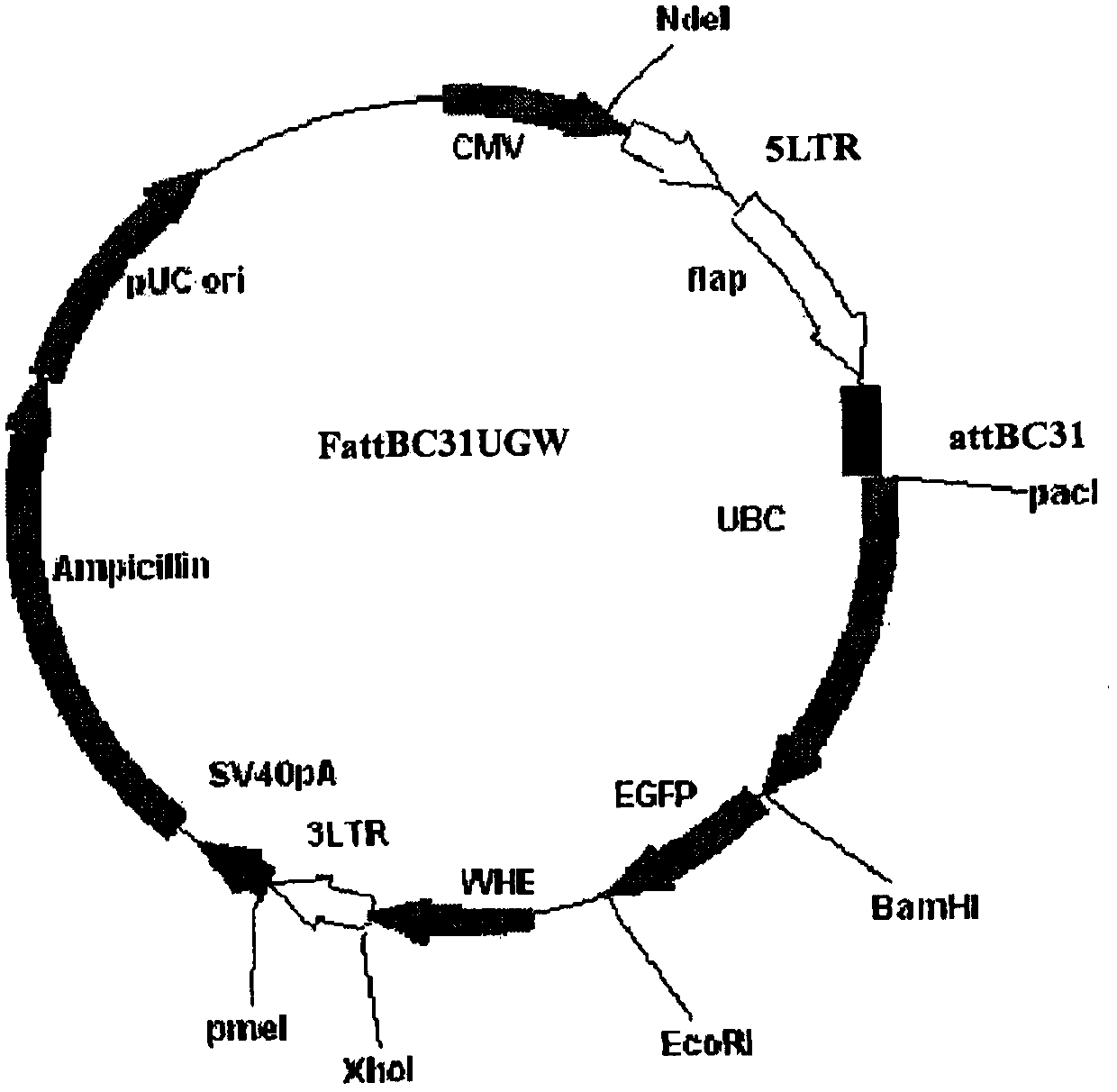
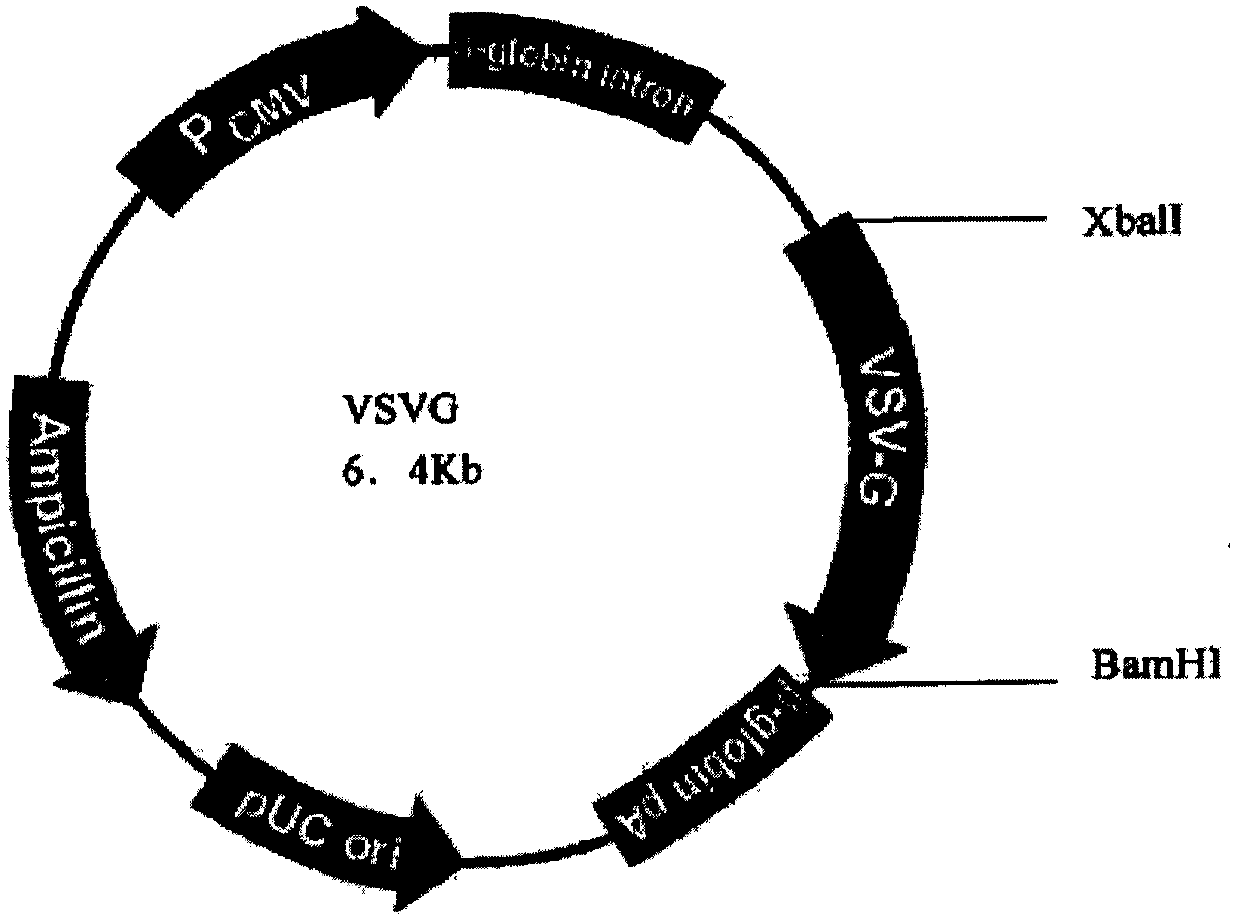
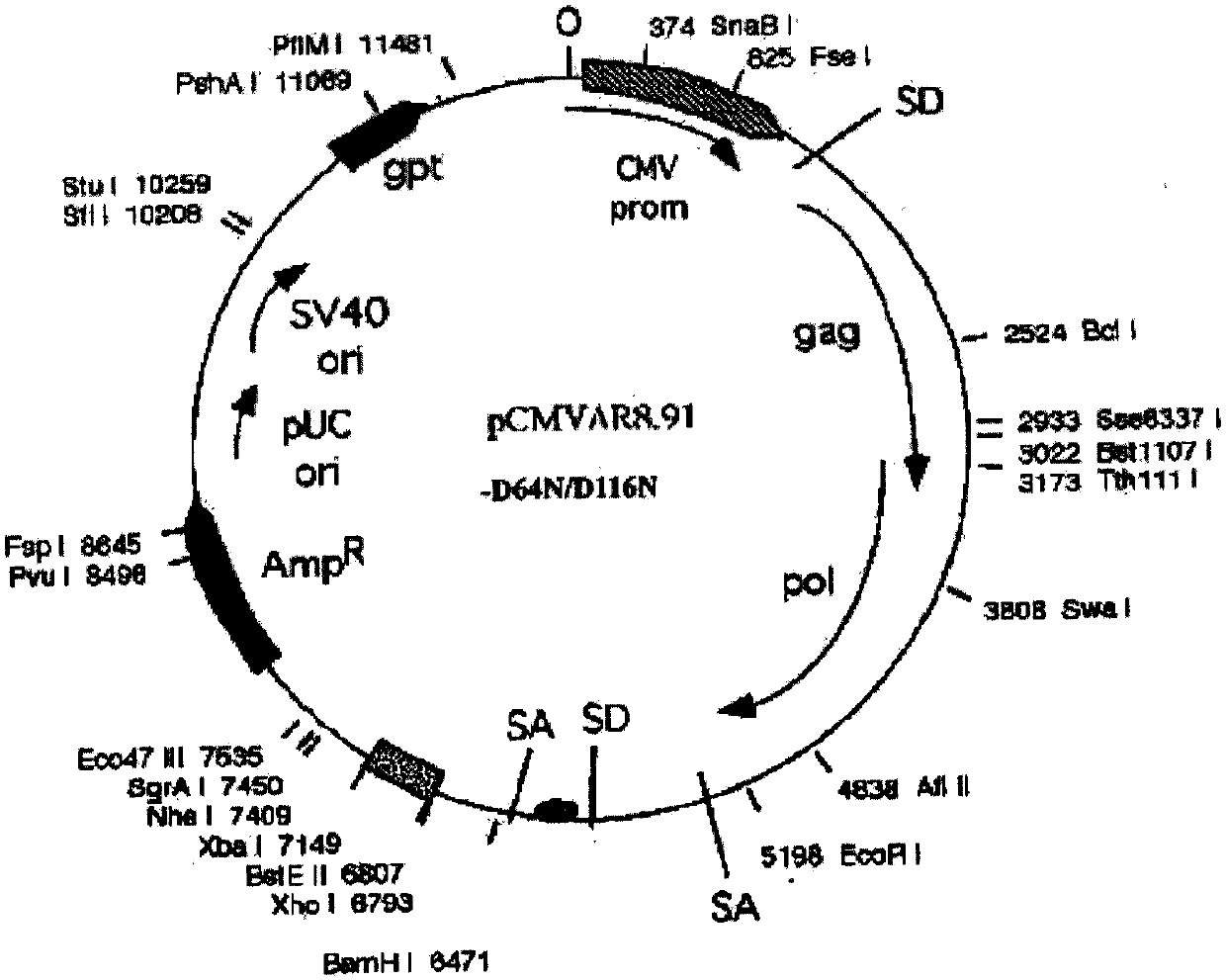
[0070] PCR primers were provided for the CDS region sequence of human peripheral blood hGM-CSF gene (GeneID: NM_000758.3), and restriction sites were added to the 5' and 3' ends of the above gene by PCR amplification, and the plasmid pORFhGM-CSF (purchased from Invivogen, article number: porf-hgmcsf) was used as a template, and the hGM-CSF gene fragment was amplified by the PCR method; the hGM-CSF gene was directional cloned into the lentiviral ligated vector system ( figure 1 Shown) to construct the recombinant vector FattbC31UGW-hGM-CSF. The recombinant vector FattbC31UGW-hGM-CSF and envelope plasmid VSVG ( figure 2 shown) and the packaging structure plasmid CMVΔ8.9-D64N / D116N ( image 3 shown) mixed with a mixing ratio of 1.5-10:1-5:1 to form a lentiviral vector system, transfected on 293T cells with liposome LipofectamineTM, observed under a fluorescence microscope after 24-48 hours, and a large amount o...
Embodiment 2
[0077] Example 2 Synthesis of Multi-target Compound Antigen Peptides
[0078] The polypeptide shown in SEQ ID NO: 5 was entrusted to Shanghai Qiangyao Biotechnology Co., Ltd. to synthesize it using a standard Fmoc protocol, and to use high performance liquid chromatography for purification and purity analysis, and mass spectrometry for identification and molecular weight determination. The result shows that the purity of the polypeptide is higher than 95%, and the molecular weight is consistent with the theoretical value.
[0079] Specific synthesis steps: 1. Fmoc-protected columns and monomers must be protected by an alkaline solvent (such as piperidine) to remove the amino protection group; 2. Use an activator to activate and dissolve the carboxyl end of the amino acid that needs to be cross-linked, and convert the activated mono The body and the free amino group are cross-linked under the action of a cross-linking agent to form a peptide bond; 3. The first two steps are rep...
Embodiment 3
[0080] Example 3 Synthesis of Multi-target Compound Antigen Peptides
[0081] The polypeptide shown in SEQ ID NO: 10 was entrusted to Shanghai Qiangyao Biotechnology Co., Ltd. to synthesize it using a standard Fmoc protocol, and to use high performance liquid chromatography for purification and purity analysis, and mass spectrometry for identification and molecular weight determination. The result shows that the purity of the polypeptide is higher than 95%, and the molecular weight is consistent with the theoretical value. Specific synthesis steps: 1. Fmoc-protected columns and monomers must be protected by an alkaline solvent (such as piperidine) to remove the amino protection group; 2. Use an activator to activate and dissolve the carboxyl end of the amino acid that needs to be cross-linked, and convert the activated mono The body and the free amino group are cross-linked under the action of a cross-linking agent to form a peptide bond; 3. The first two steps are repeated un...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com