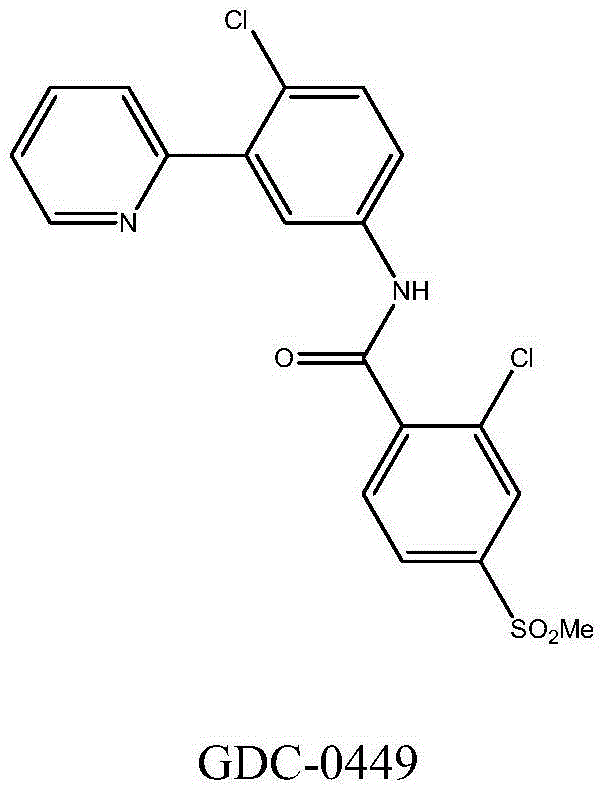
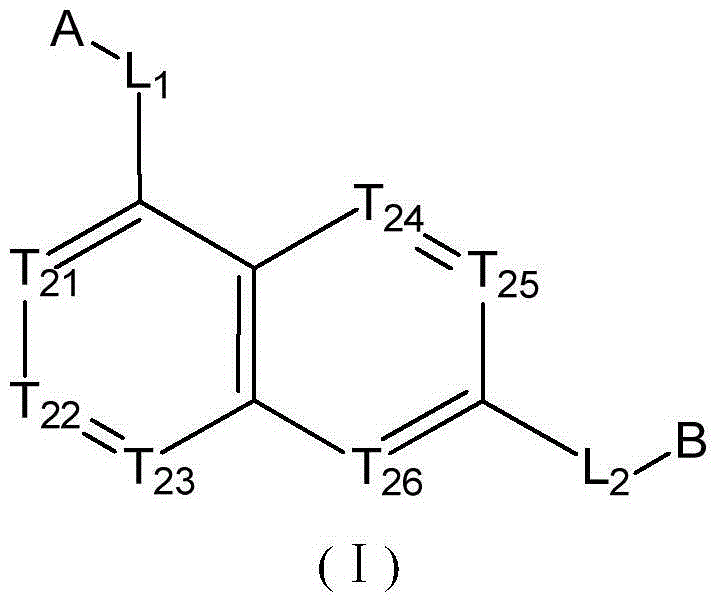
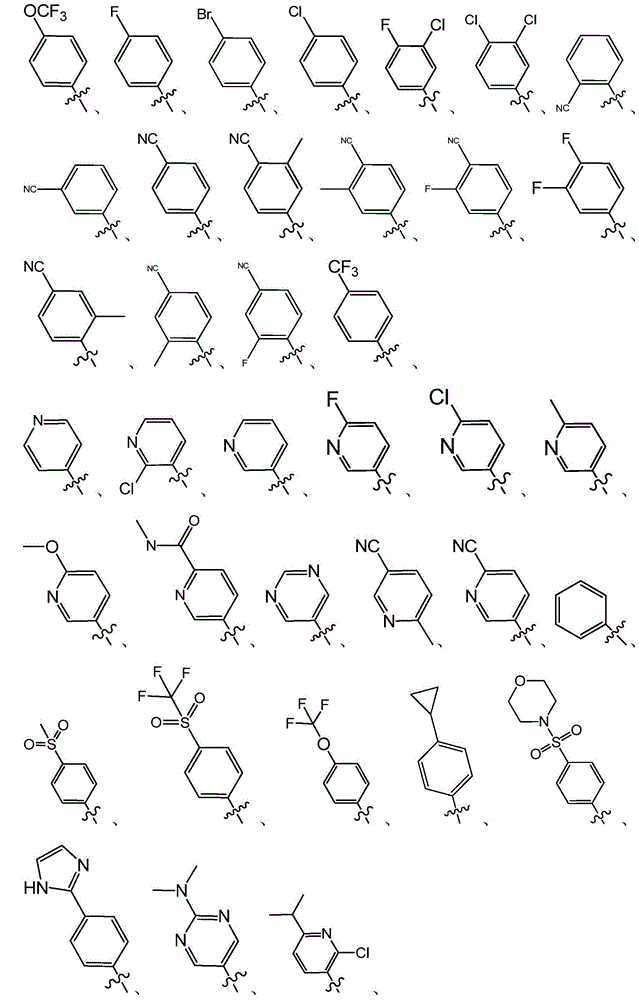
Quinoline derivatives used as SMO inhibitors
A kind of compound, selected technology, applied in the field of quinoline derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0238]
[0239] 4-(2-(4-((3R,5R)-3,5-dimethylpiperidin-1-yl)phenyl)-4-methyl-1-oxo-1,2-dihydroiso Quinolin-5-yl)benzonitrile
[0240]
[0241] Step 1: Dissolve compound 1-1 (60g, 0.36mol) in ice water (900mL), acetone (300mL) and aqueous HCl (180mL, 2.23mol), and slowly add it dropwise to sodium nitrite (50g, 0.72mol) in aqueous solution (360mL), the temperature was kept at 0-10°C. After stirring for 2 hours, solid potassium iodide (120g, 0.72mol) was added directly, and the temperature was kept at 7-10°C for 30 minutes, then the reaction solution was heated to 80-90°C until the purple gas disappeared, and cooled to room temperature. The mixture was filtered to obtain compound 1-2 (85 g, yield 85%) as a yellow solid. MS ESI calculated value C 8 h 7 IO 3 [M+H] + 279, the measured value is 279.
[0242] Step 2: Dissolve compound 1-2 (34.5g, 0.12mol) in DCM (300mL), add DMF (0.1mL), then add compound 1-3 (12mL, 0.135mol) dropwise, and stir the reaction solution for 1...
Embodiment 14
[0256]
[0257] 4-(2-(4-((3R,5R)-4-benzoyl-3,5-dimethylpiperazin-1-yl)phenyl)-4-methyl-1-oxo-1 ,2-Dihydroisoquinolin-5-yl)benzonitrile
[0258]
[0259] Step 1: Compound 14-1 (75g, 0.4mol) and triethylamine (60mL, 0.45mol) were dissolved in THF (500mL) solution and cooled to -30°C, and isobutyl chloroformate (54mL, 0.42mol) A solution in THF (100 mL) was added dropwise and the reaction was stirred at -30 °C for 0.5 h, then allowed to warm to room temperature, then stirred for an additional 5 h. The reactant was cooled to 0°C again, and the Bn 2 NH (88 mL, 0.43 mol) and TEA (70 mL, 500 mmol) were dissolved in THF (100 mL) and added dropwise, and the reactant was stirred at room temperature for 10 hours. The reaction mixture was checked by LC-MS. The mixture was poured into water and extracted with ethyl acetate, the organic layer was washed with brine, dried over sodium sulfate and concentrated under reduced pressure to give compound 14-2 as a white solid (84 g, yield 60...
Embodiment 33
[0275]
[0276] 4-(4-Methyl-1-oxo-2-(4-(3,4,5-trimethylpiperazin-1-yl)phenyl)-1,2-dihydroisoquinoline-5 -yl)benzonitrile
[0277]
[0278] Compound 33-1 (200mg, 0.45mmol), formaldehyde (41mg, 1.35mmol) and NaBH 3 CN (43 mg, 0.675 mmol) was dissolved in THF (5 mL), and the reaction solution was stirred at room temperature for 16 hours. The reaction mixture was checked by LC-MS. The crude product was purified by preparative HPLC to afford the title compound as a white solid. 1 H NMR (400MHz, DMSO-d6) δ8.43 (d, J = 8.0Hz, 1H), 7.91 (d, J = 8.0Hz, 2H), 7.61-7.54 (m, 4H), 7.35 (d, J = 8.8Hz, 2H), 7.19(s, 1H), 7.14(d, J=8.4Hz, 2H), 4.01(d, J=12.8Hz, 2H), 3.35(d, J=6.4Hz, 2H), 2.85 (t, J=12.4Hz, 2H), 1.52(s, 3H), 1.34(d, J=6.4Hz, 6H), 1.19(t, J=6.4Hz, 3H). MS ESI calculated value C 30 h 30 N 4 O[M+H] + 463, the measured value is 463.
[0279] Compounds shown in Table 3 can be synthesized from compound 33-1 and the corresponding aldehyde
[0280]
[0281]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com