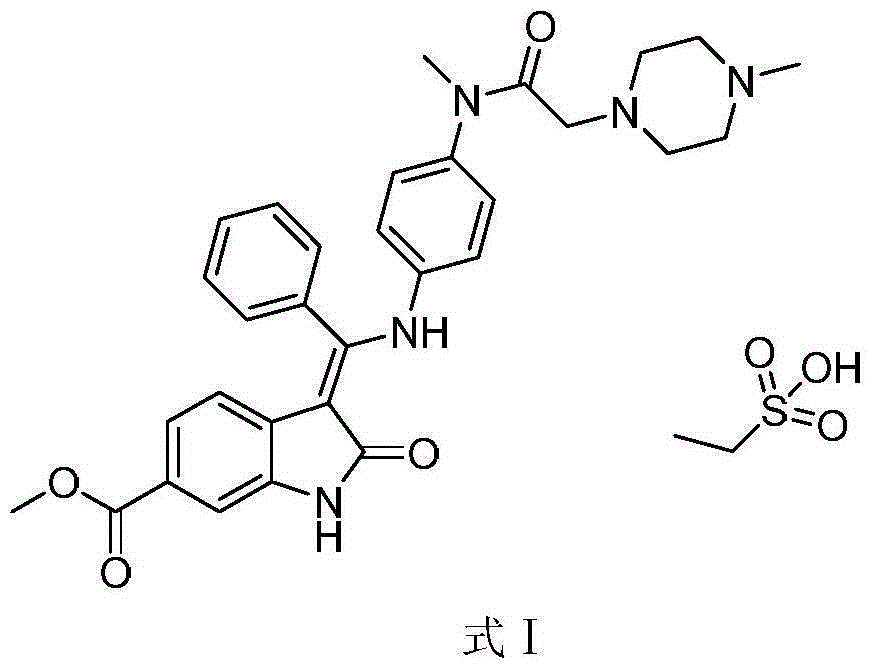
Method for preparing high-purity ethanesulfonic acid nintedanib
A technology of nintedanib ethanesulfonate and nintedanib ethanesulfonate, applied in the direction of organic chemistry, etc., can solve the problems that the purity results of nintedanib ethanesulfonate have not been disclosed, and no reports have been seen.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The refining effects of different solvents (commonly used recrystallization solvents) in the same refining process were investigated.
[0024] Dissolve the crude product of nintedanib ethanesulfonate (refer to Chinese patent CN101883755 (patent authorization announcement number) for the preparation method) in different solvents, stir and heat up to reflux until all nintedanib ethanesulfonate in the system is dissolved . After the solid is dissolved, the temperature is lowered under stirring. After the temperature is lowered to 29°C to 31°C, the finished solid of nintedanib ethanesulfonate is obtained by direct filtration.
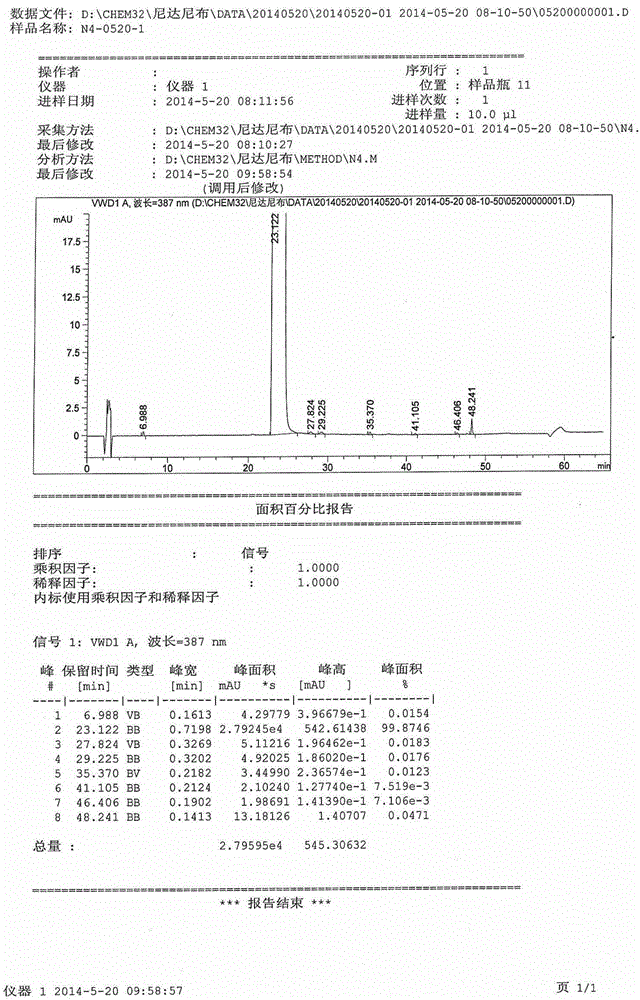
[0025] Please refer to the table below for the inspection items and results:
[0026]
[0027] Conclusion: Under the same refining operation process, the refining effect of methanol is the best, and the effect of removing the largest single impurity is the strongest. Other solvents have basically no impurity removal effect, and the maximum single...
Embodiment 2
[0029] Investigate the effect of different methanol usage on the refining effect under the same refining operation process.
[0030] Dissolve the crude product of nintedanib ethanesulfonate (refer to Chinese patent CN101883755 (patent authorization announcement number) for the preparation method) in different proportions of methanol, stir and heat up to reflux until all nintedanib ethanesulfonate in the system dissolve clear. After the solid is dissolved, the temperature is lowered under stirring. After the temperature is lowered to 25°C to 35°C, the finished solid of nintedanib ethanesulfonate is obtained by direct filtration.
[0031] Please refer to the table below for the inspection items and results:
[0032]
[0033] Conclusion: Under the same refining operation process, when the ratio of crude product:methanol is lower than 1:2.5, there is basically no impurity removal effect; the effect of removing the largest single impurity is obvious when the ratio of crude prod...
Embodiment 3
[0035] Investigate the effect of different filtration temperatures after cooling down on the refining effect under the same refining operation process.
[0036] Dissolve the crude product of nintedanib ethanesulfonate (refer to Chinese patent CN101883755 (patent authorization announcement number) for the preparation method) in methanol of the same proportion, stir and heat up to reflux until all nintedanib ethanesulfonate in the system dissolve clear. After the solid was dissolved, the temperature was lowered under stirring, and after cooling to different temperatures, the finished solid of nintedanib ethanesulfonate was obtained by direct filtration, and the solid was filtered to remove the solvent and then sampled for testing.
[0037] Please refer to the table below for the inspection items and results:
[0038]
[0039] Conclusion: Under the same refining process, different filtration temperatures have a great influence on the refining effect. When the filtration temp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com