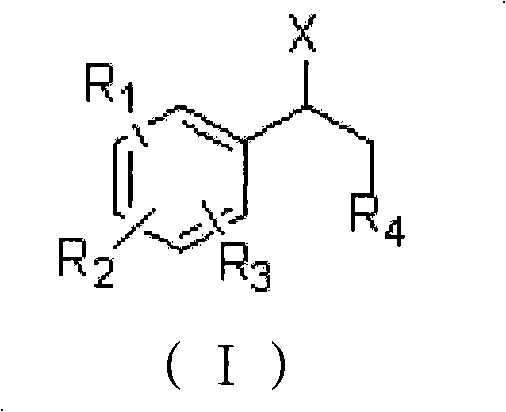
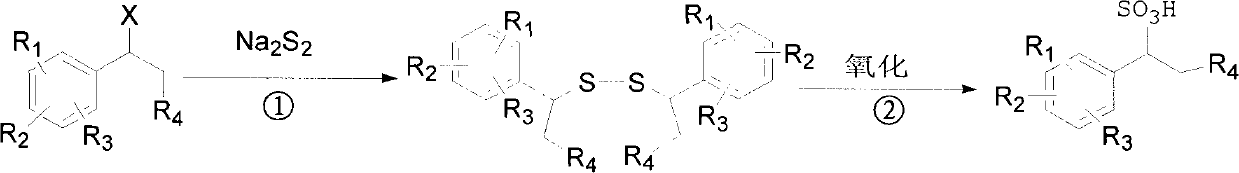
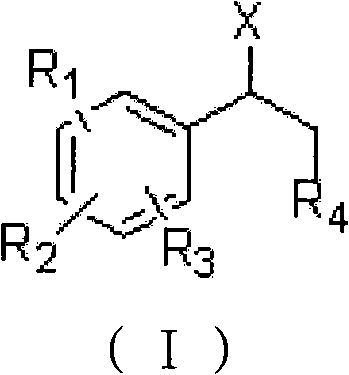
Method for preparing 1-aryl ethanesulfonic acid
A technology of arylethanesulfonic acid and arylhaloethane, which is applied in the field of preparation of 1-arylethanesulfonic acid, can solve the problem of unpleasant odor of mercaptan, difficulty in separation, and difficulty in 1-phenyl Ethanesulfonic acid, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 55g (0.5mol) of sodium disulfide and 200mL of ethanol to a four-necked flask equipped with a stirrer, a condenser, and a thermometer, heat and stir until the sodium disulfide dissolves, then slowly add 185g (1mol) of 1-bromophenylethane dropwise. ). After the dropwise addition, the temperature was raised to reflux temperature for reaction, and the reaction was tracked by thin-layer chromatography until the end of the reaction. After the reaction was completed, the mixture was left standing to separate layers, and the organic layer was concentrated to obtain 1-phenylethyl disulfide, which weighed 123.3 g and had a yield of 90%.
[0030] Add 123.3g of 1-phenethyl disulfide to the three-necked flask, then add 162g of acetic acid, and add 50% H 2 o 2 200ml, stirred at room temperature, followed by TLC until the end of the reaction. 1-Phenylethanesulfonic acid was determined by HPLC to obtain 160.7 g, and the yield was 96%.
[0031] HPLC conditions: chromatographic c...
Embodiment 2
[0036] In a four-necked flask equipped with a stirrer, a condenser, and a thermometer, add 40 g (0.36 mol) of sodium disulfide and 40 g of water, heat and stir until the sodium disulfide is completely dissolved, then add 1.5 g of tetrabutylammonium bisulfate, slowly 135.05 g (0.73 mol) of 1-bromophenylethane was added dropwise. After the dropwise addition, the reaction was carried out at the reflux temperature, and the reaction was tracked by thin-layer chromatography until the end of the reaction. After the reaction was completed, it was extracted with petroleum ether, dried, filtered, and concentrated to obtain 89.0 g of 1-phenethyl disulfide, with a yield of 89%.
[0037] Add 89.0 g of 1-phenethyl disulfide to the three-necked flask, and add 5% KMnO dropwise at room temperature 4 2000.9g, the dropwise addition was completed, and the reaction was maintained at room temperature, and the reaction was tracked by thin-layer chromatography until the end of the reaction. 1-Pheny...
Embodiment 3
[0039] In a 250mL four-neck flask equipped with a stirrer, condenser, and thermometer, add 120.1g (0.5mol) of sodium sulfide (containing 9 crystal water), 9g (0.5mol) of water, heat and stir until the sodium sulfide is completely dissolved , add sulfur 16g (0.5mol), continue the reaction at reflux temperature for 1h, to obtain sodium disulfide solution.
[0040] Sodium disulfide solution was slowly added to a solution of 185 g (1 mol) of 1-bromophenylethane and 0.4 g of tetrabutylammonium bisulfate, and reacted at reflux temperature to generate 1-phenethyl disulfide. After the reaction was completed, the layers were separated, and the organic layer was 1-phenethyl disulfide to obtain 120.6 g. The yield was 88%.
[0041] Add 120.6g of 1-phenethyl disulfide to the three-necked flask, then add 212mL of acetic acid, add 50% H 2 o 2 239.4mL, stirred at room temperature for 12h, and the reaction ended. 1-phenylethanesulfonic acid was determined by HPLC to obtain 155.5 g, and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com