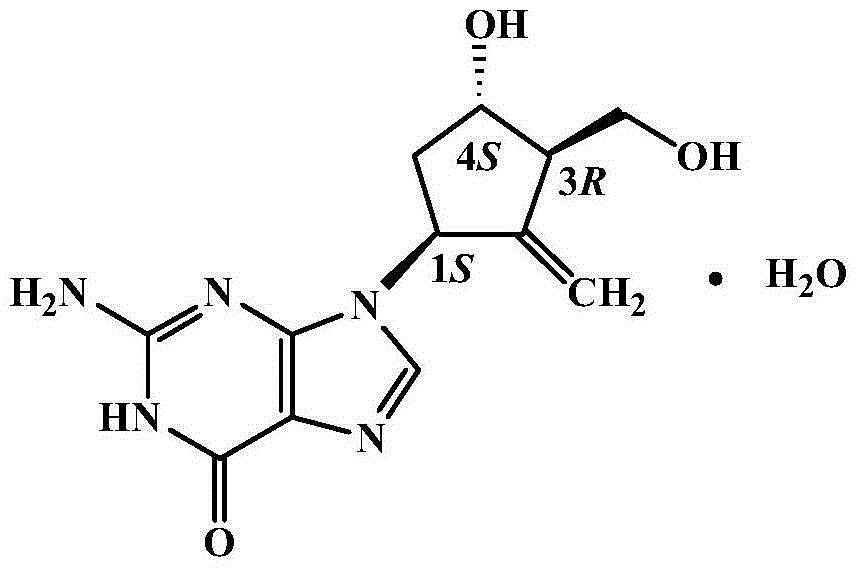
Entecavir crystalline compound and capsule preparation thereof
A crystalline compound, entecavir technology, applied in the direction of active ingredients of heterocyclic compounds, capsule delivery, organic chemistry, etc., can solve the problems of slow dissolution rate and slow onset of action, and achieve long maintenance time, fast release speed, and improved water solubility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
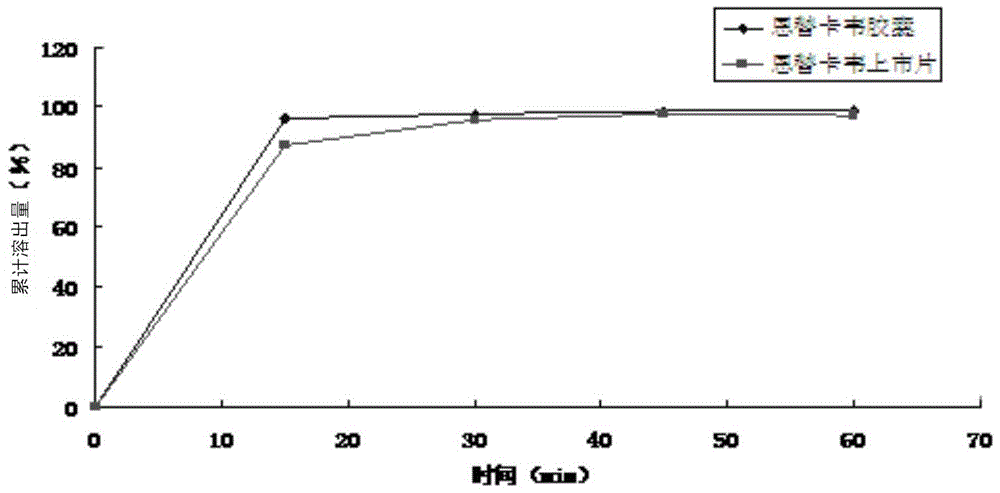
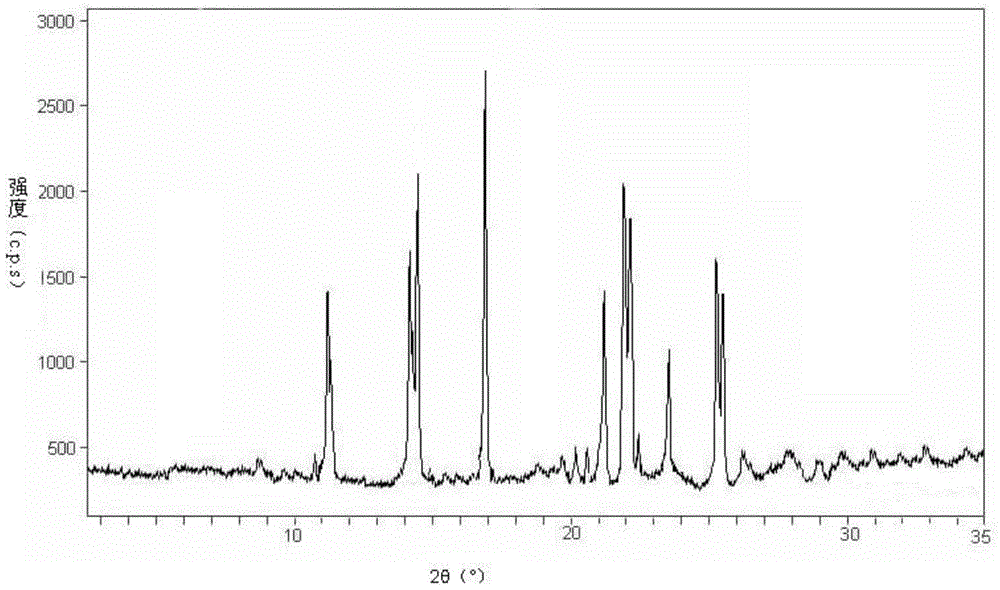
[0041] Add 10g of entecavir solid into 100ml of mixed solvent of dimethylacetamide and methanol, the volume between dimethylacetamide and methanol is 2.0:1.5, and the temperature is at 40°C, all the entecavir solids are dissolved; control the temperature at Under the condition of 35~40 ℃, add the aqueous solution of isopropanol in the obtained solution, wherein, the mass percent of isopropanol is contained in the aqueous solution of isopropanol is 35%; After adding the aqueous solution of isopropanol, at a rate of Cool down to -5°C at 3.5°C / 10min, stand at -5°C for 13 hours, precipitate crystals, filter, wash the filter cake with ether, and dry in vacuo to obtain entecavir crystals. Carry out Cu-Kα to the obtained product entecavir crystal 1 Radiographic X-ray powder diffraction (eg figure 1 Shown), in 11.1 °, 13.9 °, 14.4 °, 21.1 °, 21.8 °, 22.1.9 °, 23.5 °, 25.2 °, 25.4 °, ° diffraction angle (2θ ± 0.1) shows diffraction peaks.
Embodiment 2
[0043] Add 15g of entecavir solid into 100ml of mixed solvent of dimethylacetamide and methanol, the volume between dimethylacetamide and methanol is 2.0:2.0, and the entecavir solid is completely dissolved at 35°C; control the temperature at Under the condition of 35-40°C, add an aqueous solution of isopropanol to the obtained solution, wherein the aqueous solution of isopropanol contains 30% by mass of isopropanol ethanol; after adding the aqueous solution of isopropanol, at a rate Cool down to -10°C at 2.5°C / 10min, stand at -10°C for 18 hours, precipitate crystals, filter, wash the filter cake with ether, and vacuum dry to obtain entecavir crystals. Show through powder XRD detector analysis, with attached figure 1 The results shown match.
Embodiment 3
[0045] Add 12 solids of Entecavir into 100ml of mixed solvent of dimethylacetamide and methanol, the volume between dimethylacetamide and methanol is 2.0:2.0, and the temperature is at 40°C, all solids of Entecavir are dissolved; control the temperature at Under the condition of 35-40°C, add an aqueous solution of isopropanol to the obtained solution, wherein the aqueous solution of isopropanol contains isopropanol with a mass percentage of 40% ethanol; after adding the aqueous solution of isopropanol, at a rate Cool down to 0°C at 3.0°C / 10min, stand at 0°C for 20 hours, precipitate crystals, filter, wash the filter cake with ether, and dry in vacuo to obtain entecavir crystals. Show through powder XRD detector analysis, with attached figure 1 The results shown match.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap