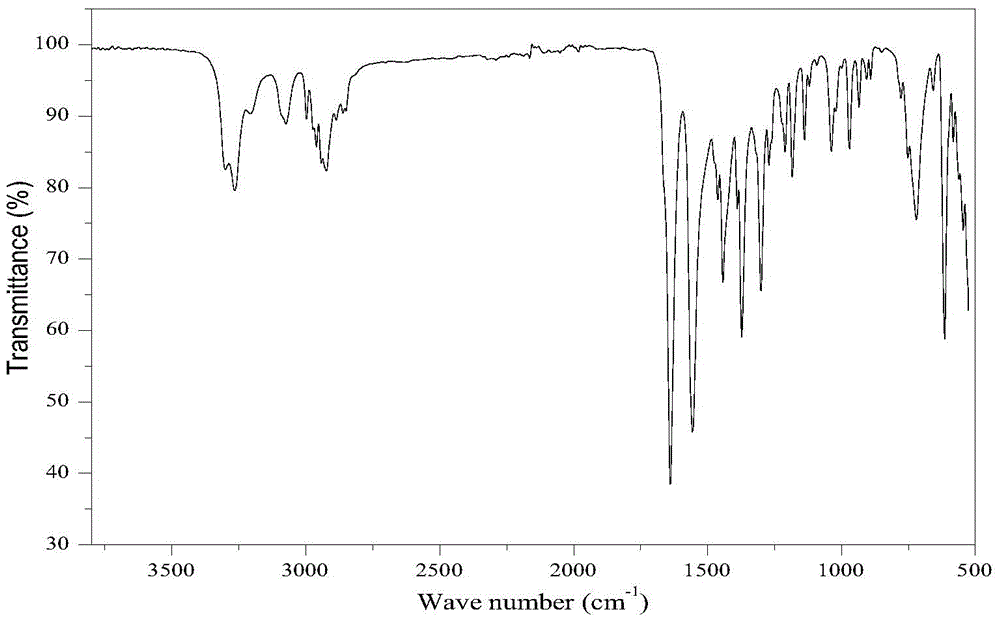
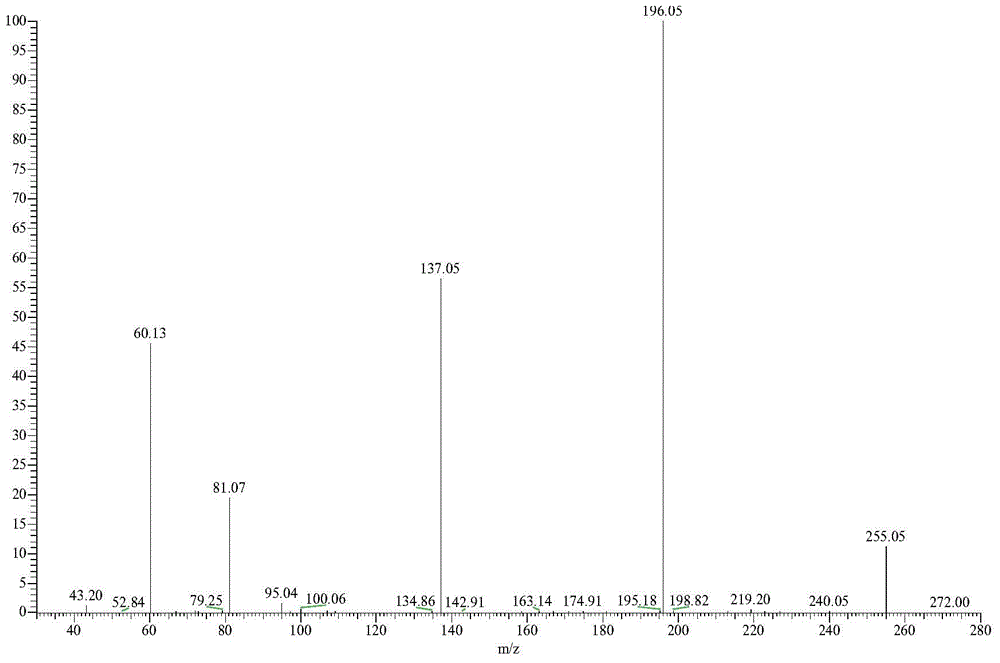
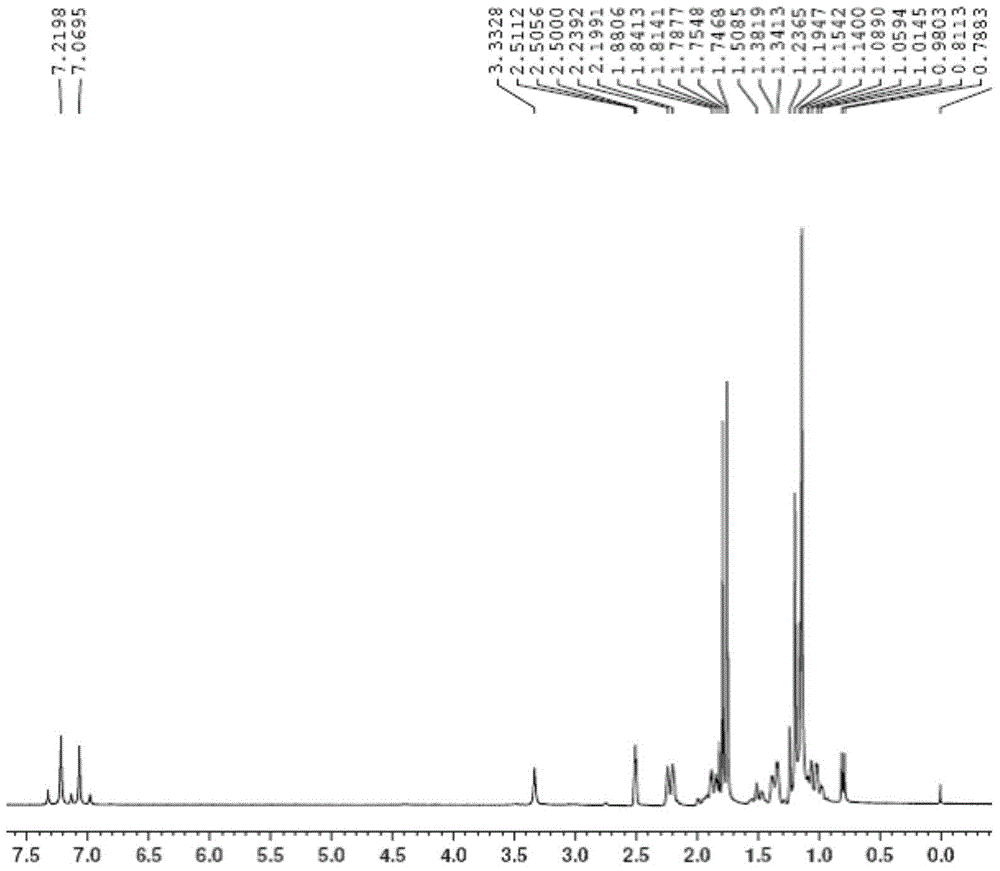
Method for preparing 1,8-menthane diacetyl amide from turpentine
A technology of menthane diacetamide and turpentine, applied in the field of producing 1,8-mentane diacetamide, which can solve the problems of low efficiency, high cost, cumbersome purification process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 84.3g turpentine (94.5% pinene GC content, 0.58mol) and 63.2g acetonitrile (1.54mol) into a 1L reactor, mechanically stir until the solution is evenly mixed, slowly drop 225g60% H 2 SO 4 Aqueous solution (containing H 2 SO 4 1.38mol), after the dropwise addition was completed, the temperature was slowly raised to 75°C, and the heat preservation reaction was carried out for 5 hours. After the reaction was completed, the reaction solution was neutralized to neutrality with aqueous sodium hydroxide solution, extracted with ethyl acetate, and the organic layer was collected to precipitate the product , filtered and washed the precipitate to obtain 91.1 g (0.36 mol) of 1,8-mentane diacetamide.
Embodiment 2
[0034] Add 84.6g turpentine (94.5% pinene GC content, 0.59mol) and 79.0g acetonitrile (1.92mol) into a 1L reactor, stir mechanically until the solution is evenly mixed, and slowly drop in 225g60% H 2 SO 4 Aqueous solution (containing H 2 SO 4 1.38mol), after the dropwise addition was completed, the temperature was slowly raised to 75°C, and the heat preservation reaction was carried out for 5 hours. After the reaction was completed, the reaction solution was neutralized to neutrality with aqueous sodium hydroxide solution, extracted with ethyl acetate, and the organic layer was collected to precipitate the product , filtered and washed the precipitate to obtain 41.6 g (0.16 mol) of 1,8-mentane diacetamide.
Embodiment 3
[0036]Add 84.4g turpentine (94.5% pinene GC content, 0.59mol) and 39.5g acetonitrile (0.96mol) into a 1L reactor, stir mechanically until the solution is evenly mixed, and slowly drop in 225g60% H 2 SO 4 Aqueous solution (containing H 2 SO 4 1.38mol), after the dropwise addition was completed, the temperature was slowly raised to 75°C, and the heat preservation reaction was carried out for 5 hours. After the reaction was completed, the reaction solution was neutralized to neutrality with aqueous sodium hydroxide solution, extracted with ethyl acetate, and the organic layer was collected to precipitate the product , filtered and washed the precipitate to obtain 22.3 g (0.088 mol) of 1,8-mentane diacetamide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com