Application of aphanalide I in preparation of neuroprotective medicines
A kind of technology of aphanalidei, 1.aphanalidei, be applied in the application field of Aphanalide I in the preparation of neuroprotective medicine, can solve the problems such as not having compound activity report yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Example 1: Isolation, preparation and structural confirmation of Aphanalide I
[0013] The preparation method of Aphanalide I is the same as that reported in the literature (BioactiveTerpenoids from the Fruits of Aphanamixis grandifolia, J.Nat.Prod., 2013, 76, 1191-1195).
[0014] Confirmation of structure: colorless crystal (methanol), melting point 273-274°C, molecular formula C 26 h 34 o 7 , with an unsaturation of 10. Infrared absorption is strong, indicating that it contains hydroxyl groups (3466cm -1 ), carbonyl (1729cm -1 ), and alkenes (1683cm -1 ) and other groups. It has ultraviolet absorption at 230nm, indicating that it contains α, β unsaturated ester carbonyl moiety. H NMR spectrum data δ H (ppm, DMSO-d 6 , 500MHz): H-1 (6.75, d, J=12.0), H-2 (5.79, d, J=12.0), H-5 (2.57, d, J=13.0), H-6 (1.45, dt , J=13.0, 3.5), H-6 (1.93, t, J=13.0), H-7 (3.50, s), H-9 (1.83, s), H-11 (4.22, s), H- 12 (3.56, t, J=4.5), H-14 (2.85, s), H-16 (2.56, dd, J=19.0, 11...
Embodiment 2
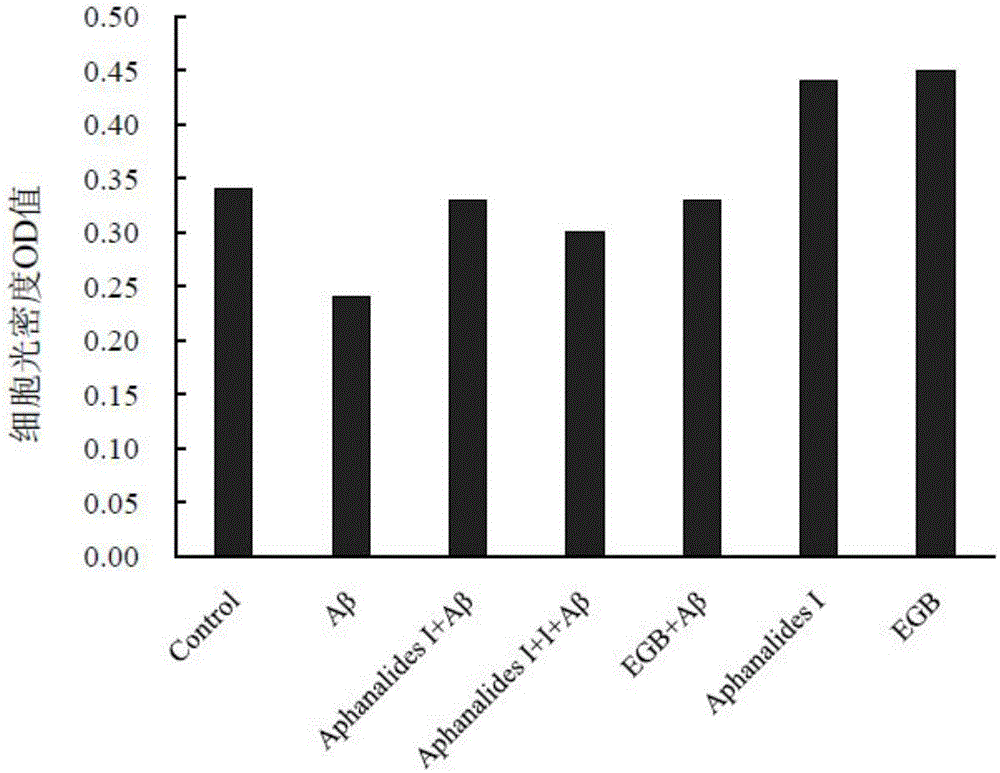
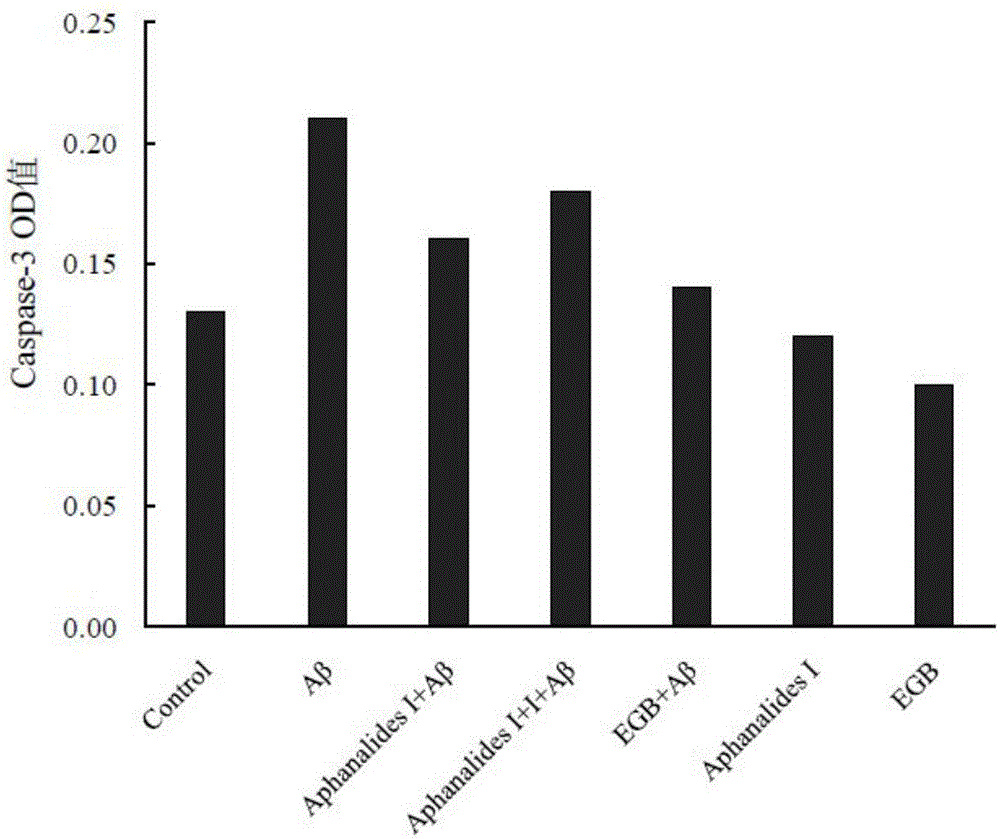
[0015] Embodiment 2: the pharmacological action test of Aphanalide I
[0016] 1. Materials and Instruments
[0017] Healthy female SD rats were provided by the Experimental Animal Center of Shanghai Second Military Medical University. AphanalideI is self-made, and the HPLC normalized purity is greater than 98%. Dimethyl sulfoxide, ginkgo extract (EGb761, positive drug), MTT, Aβ25-35, and L-polylysine were all purchased from SIGMA, USA. DMEM high-glucose medium, Neurobasal, and B27 were purchased from GIBCO, USA. NSE was purchased from ABCOM, UK. Tunel was purchased from Wuhan Boster Company. LDH kit was purchased from Nanjing Jiancheng Biochemical Reagent Company.
[0018] Electronic balance, Beijing Sartorius. 1815TCCO 2 Constant temperature incubator (Shel-Lab, USA), TH-2C constant temperature oscillator (Jiangsu Taicang Experimental Equipment Factory), dissecting microscope (OLYMPUS, Japan), desktop centrifuge (Heraeus, Germany), microplate reader (DynaTtech, Japan) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| degree of unsaturation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com