A kind of preparation method of dapoxetine hydrochloride
A technology of dapoxetine hydrochloride and hydrochloride, which is applied in the field of medicine, can solve the problems of increased synthesis cost, product loss, and tediousness, and achieve the effects of reducing production cost and loss, short synthesis route, and cost saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
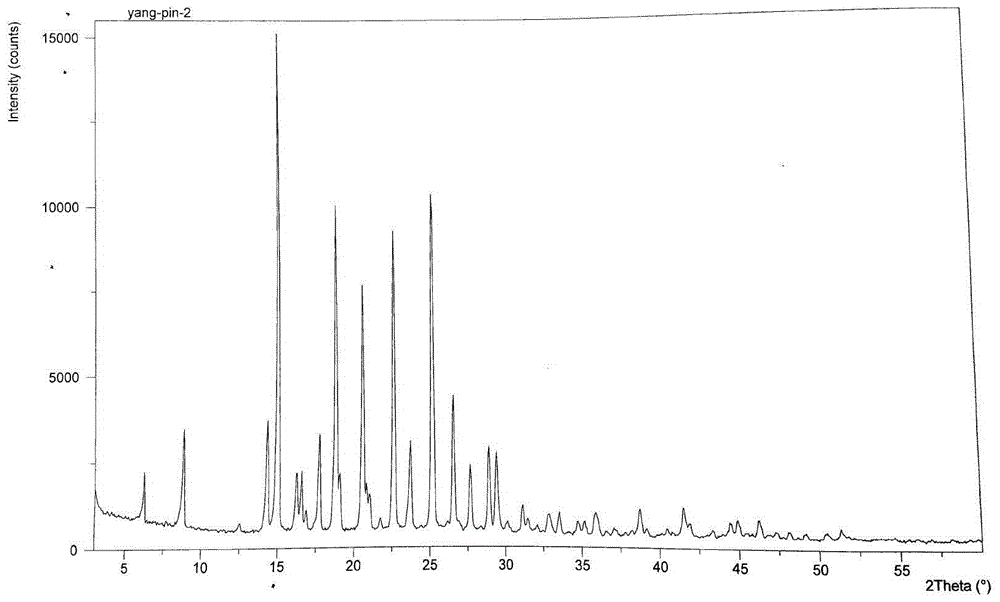
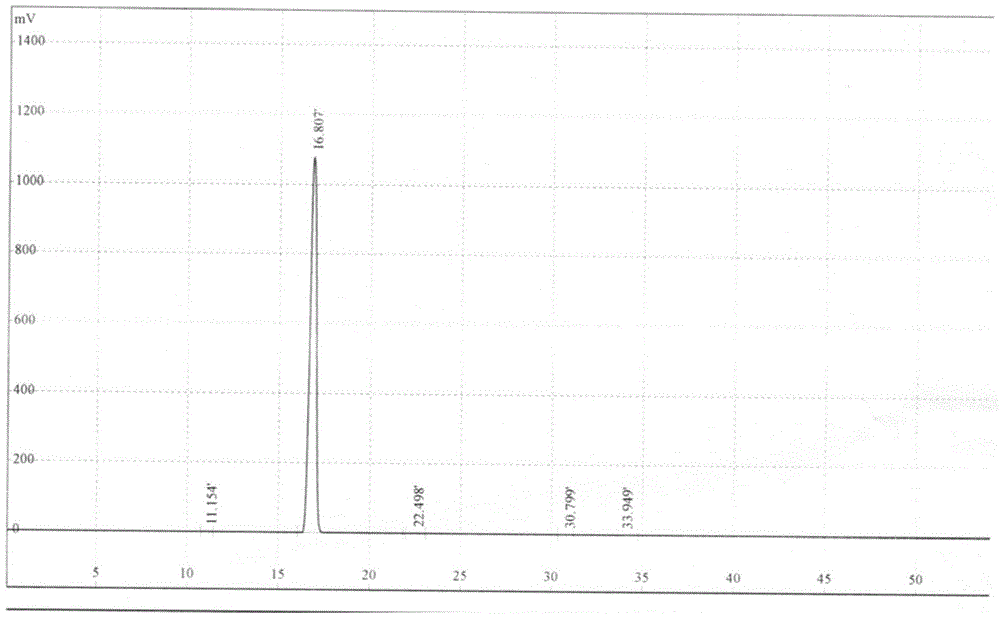
Image
Examples
specific Embodiment approach
[0059] The following examples are used to illustrate the present invention, but are not intended to limit the scope of the present invention. The NaBH used in the following implementation 4 -ZnC1 2 The system is prepared by the following preparation method:
[0060] (1) Commercially available zinc chloride solids are heated and melted on an electric furnace to remove crystal water inside to prevent its reducing effect, and then cooled to obtain anhydrous zinc chloride;
[0061] (2) Add 30mL of anhydrous tetrahydrofuran to a 100mL single-necked bottle. At room temperature, take anhydrous zinc chloride (40.12, 0.295mol) and dissolve it in anhydrous tetrahydrofuran, add sodium borohydride (22.4g, 0.59mol) ), stirred for 2h to obtain a white suspension for use.
Embodiment 1
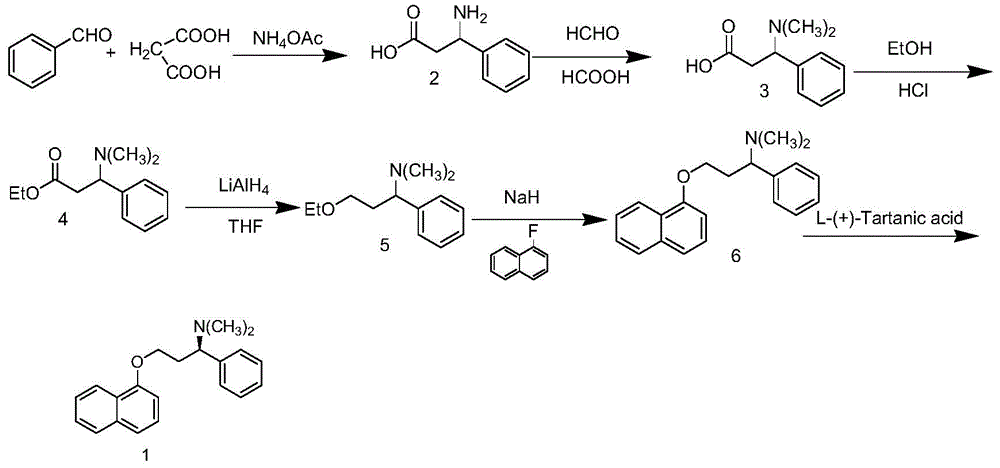
[0063] The present embodiment is a kind of preparation method of dapoxetine hydrochloride, specifically comprises the following steps:
[0064] (1) Preparation of Intermediate II
[0065]
[0066] Add 57.6g (0.267mol) of chiral L-phenylalanine methyl ester hydrochloride (I) into a 1000ml three-necked reaction flask, add 300ml of anhydrous THF and stir, then suspend the newly prepared sodium borate-zinc chloride The solution was slowly added dropwise to the reaction system. After the dropwise addition, the temperature was raised to reflux for 4 hours, and the reaction progress was tracked by TLC until the reaction of the raw materials was completed. Cool down to 30-35°C and add 255ml of methanol dropwise. After the dropwise addition, reflux for 30min, cool down, filter, and concentrate the filtrate under reduced pressure to obtain 47.1g of white solid (Intermediate II), with a yield of 99.6%.
[0067] (2) Preparation of intermediate III
[0068]
[0069] Add 44.25g (0.2...
Embodiment 2
[0074] The difference between this embodiment and Example 1 is that the preparation method of intermediate II in step (1) is different: in this embodiment, L-phenylalanine butyl ester hydrochloride is used as the reaction raw material, and its addition is also 0.267mol, and finally obtained 46.3g of intermediate II with a yield of 97.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com