Fully human anti-RANKL antibody
An antibody and whole-person technology, applied in the direction of antibodies, anti-inflammatory agents, anti-tumor drugs, etc., can solve problems such as increased difficulty, difficult production control, difficult quality control, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Example 1: Design and expression of BA05-1 and BA05-2 antibody sequences
[0076] The following is the sequence of the heavy chain variable region of AMG162:
[0077] EVQLLESGGGLVQPGGSLRLSCAASGFTFSSYAMSWVRQAPGKGLEWVSGITGSGGSTYYADSVKGRFTISRDNSKNTLYLQMNSLRAEDTAVYYCAKDPGTTVIMSWFDPWGQGTLVTVSS (SEQ ID NO: 9)
[0078] The following is the heavy chain constant region sequence of AMG162:
[0079] ASTKGPSVFPLAPCSRSTSESTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSNFGTQTYTCNVDHKPSNTKVDKTVERKCCVECPPCPAPPVAGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVQFNWYVDGVEVHNAKTKPREEQFNSTFRVVSVLTVVHQDWLNGKEYKCKVSNKGLPAPIEKTISKTKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPMLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK(SEQIDNO:10)
[0080] The original heavy chain IgG2 constant region sequence was replaced with IgG4 constant region sequence to form the BA05-1 heavy chain sequence:
[0081] EVQLLESGGGLVQPGGSLRLSCAASGFTFSSYAMSWVRQAPGKGLEWVSGITGSGGSTYYADSVKGRFTISRDNSKNTLYLQMNSLRAE...
Embodiment 2
[0094] Example 2: Expression and preparation of BA05-1 and BA05-2 antibodies
[0095] The full-length DNA sequence of the heavy chain of BA05-1 is:
[0096]GAGGTGCAGCTCCTGGAGAGCGGCGGAGGCCTGGTGCAGCCCGGAGGAAGCCTGCGGCTCTCCTGCGCCGCTAGCGGATTCACATTCTCCAGCTACGCTATGAGCTGGGTCAGGCAGGCTCCTGGCAAGGGACTCGAGTGGGTGAGCGGCATCACCGGATCCGGCGGATCCACATACTATGCCGATTCCGTCAAGGGAAGGTTCACAATCTCCCGGGACAACAGCAAGAACACCCTCTACCTCCAGATGAACAGCCTGCGGGCCGAGGACACAGCCGTCTACTATTGCGCCAAAGACCCCGGAACCACCGTGATCATGAGCTGGTTCGATCCCTGGGGACAGGGAACCCTCGTGACAGTGTCCAGCGCTAGCACCAAGGGCCCATCCGTCTTCCCCCTGGCGCCCTGCTCCAGGAGCACCTCCGAGAGCACAGCCGCCCTGGGCTGCCTGGTCAAGGACTACTTCCCCGAACCGGTGACGGTGTCGTGGAACTCAGGCGCCCTGACCAGCGGCGTGCACACCTTCCCGGCTGTCCTACAGTCCTCAGGACTCTACTCCCTCAGCAGCGTGGTGACCGTGCCCTCCAGCAGCTTGGGCACGAAGACCTACACCTGCAACGTAGATCACAAGCCCAGCAACACCAAGGTGGACAAGAGAGTTGAGTCCAAATATGGTCCCCCATGCCCACCATGCCCAGCACCTGAGTTCCTGGGGGGACCATCAGTCTTCCTGTTCCCCCCCAAACCCAAGGACACTCTCATGATCTCCCGGACCCCTGAGGTCACGTGCGTGGTGGTGGACGTGAGCCAGGAAGACCCCGAGGTCCAGTTCAACTGG...
Embodiment 3
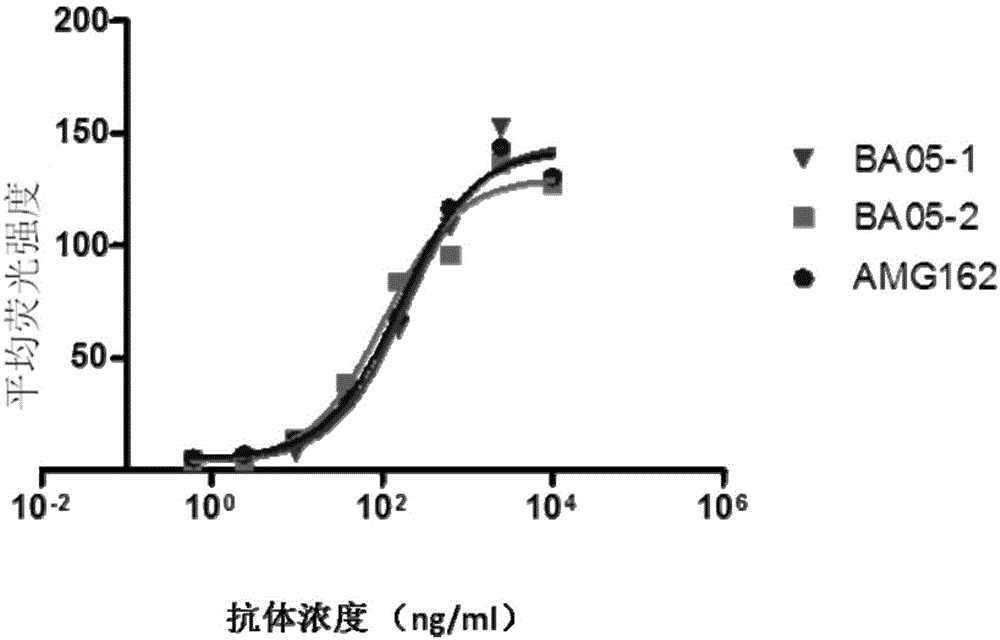
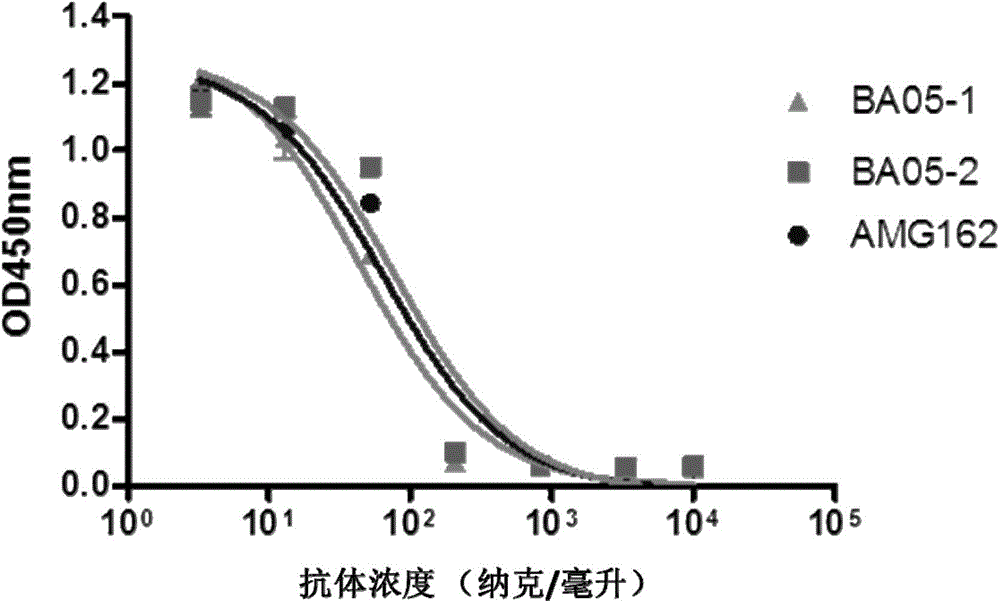
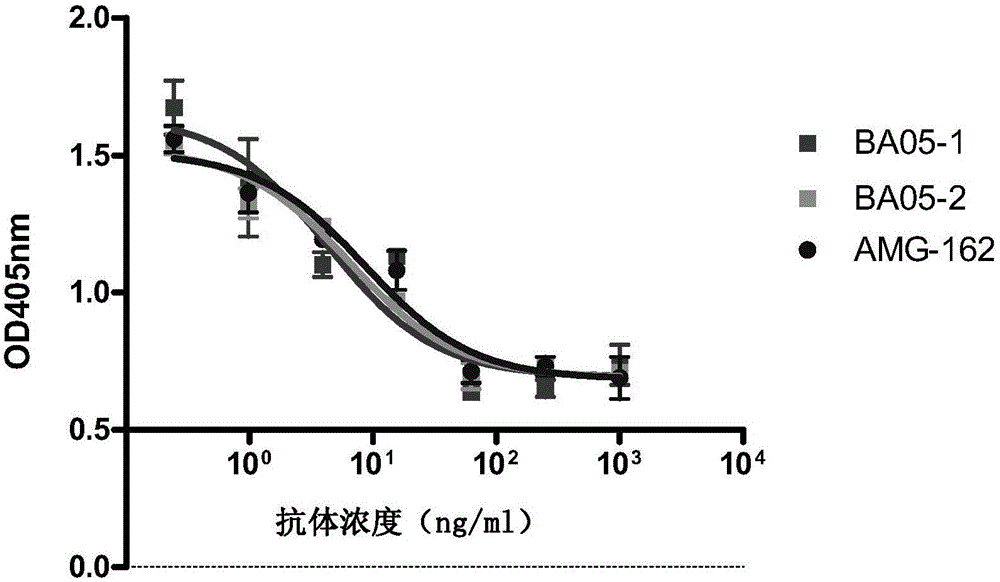
[0106] Example 3: Detection of the binding of BA05-1 and BA05-2 antibodies to RANKL by flow cytometry
[0107] Binding of anti-RANKL antibodies BA05-1 and BA05-2 to RANKL (full-length RANKL) transfected with RANKL on the surface of 293 cells (Invitrogen) was detected by flow cytometry. 293-RANKL transfected cells were incubated with different concentrations of anti-RANKL antibodies BA05-1, BA05-2, and AMG162 at 4°C for 1 h. The cells were washed with PBS, and anti-human IgG (H+L)--PE (1:200) / anti-human IgG-R-PE (1:1000) antibodies were added to incubate at 4°C for 30 min. After washing with PBS, the cells were resuspended in 1 ml of PBS and analyzed by flow cytometry (BDFACScan). Such as figure 1 As shown, the binding ability of BA05-1 to RANKL is equivalent to that of AMG162, while the binding ability of BA05-2 to RANKL is slightly better than that of AMG162.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com