Cholinolytic drug glycopyrronium bromide and composition
A technology of glycopyrronium bromide and composition, which is applied in the field of pharmaceutical compositions containing glycopyrronium bromide, and can solve problems such as no central anticholinergic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0182] Embodiment 1: Preparation of glycopyrronium bromide bulk drug
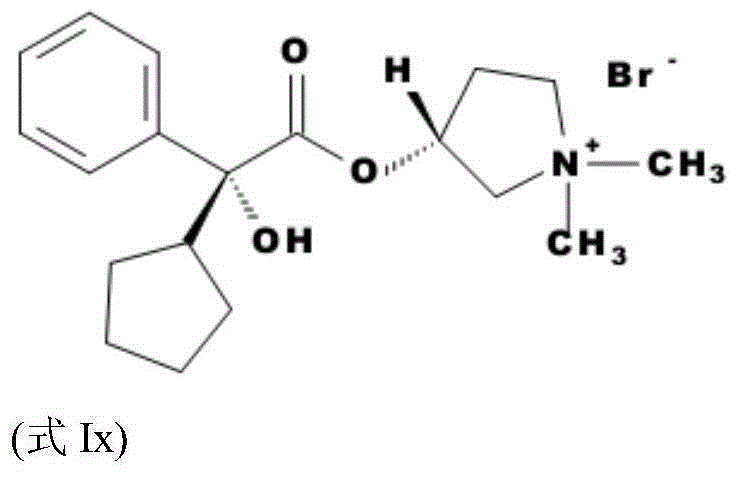
[0183] Step (1), synthetic formula I compound and enantiomer thereof:
[0184] Make hydroxycyclopentyl mandelic acid methyl ester (MCPM) and N-methylpyrrolidin-3-alcohol (NMP) reaction according to the method in US2956062A to obtain glycopyrrolate base (GP base), then according to CN101133021B specification sheet [0015]- The method contained in section makes glycopyrronium base react with methyl bromide and then carries out recrystallization refining to gained product, obtains glycopyrronium bromide.
[0185] RRT0.89 content=4.63%, RRT1.14 content=1.78%, RRT2.68 content=1.14%.
[0186] Step (2), recrystallization refining:
[0187] At a temperature of 55-60°C, dissolve 1.5 g of the product obtained in step (1) in 10 ml of anhydrous ethanol-ethyl acetate mixed solvent (100:12, v / v), and slowly add 120 ml of methyl ethyl ether dropwise to it , standing at 4-5° to allow sufficient precipitation (about 24 h...
Embodiment 2
[0200] Embodiment 2: Preparation of glycopyrronium bromide bulk drug
[0201] Step (1), synthetic formula I compound and enantiomer thereof:
[0202] The crude product of glycopyrronium bromide was prepared according to the method in the specific embodiment part of the CN103159659A description, that is, the method described in paragraph [0010] to [0013], line 4 of the description. RRT0.89 content=4.76%, RRT1.14 content=1.61%, RRT2.68 content=1.21%.
[0203] Step (2), recrystallization refining:
[0204] The product obtained in the above step (1) was recrystallized according to the method of step (2) of Example 1 of the present invention (the recrystallization yield was 89.7%).
[0205] RRT0.89 content=0.83%, RRT1.14 content=0.34%, RRT2.68 content=0.20%. In this recrystallization purification, the content of RRT0.89 was reduced by 82%, the content of RRT1.14 was reduced by 79%, and the content of RRT2.68 was reduced by 83%.
[0206] Step (3), recrystallization refining ag...
Embodiment 3
[0214] Embodiment 3: Preparation of glycopyrronium bromide bulk drug
[0215] Step (1), synthetic formula I compound and enantiomer thereof:
[0216] Glycopyrronium bromide was prepared according to the method in the specific embodiment part of the CN102627595A description, that is, the method described in paragraphs [0043] to [0046] of the description. RRT0.89 content=4.13%, RRT1.14 content=1.36%, RRT2.68 content=1.03%.
[0217] Step (2), recrystallization refining:
[0218] The product obtained in the above step (1) was recrystallized according to the method of step (2) of Example 1 of the present invention (recrystallization yield 92.4%).
[0219] RRT0.89 content=0.70%, RRT1.14 content=0.24%, RRT2.68 content=0.16%. In this recrystallization and purification, the content of RRT0.89 was reduced by 83%, the content of RRT1.14 was reduced by 82%, and the content of RRT2.68 was reduced by 84%.
[0220] Step (3), recrystallization refining again:
[0221] The product obtai...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com