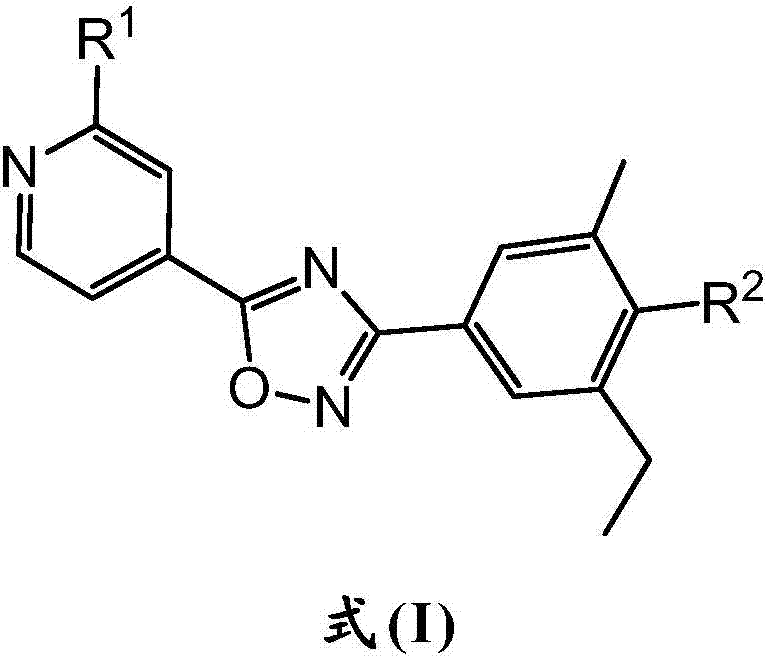
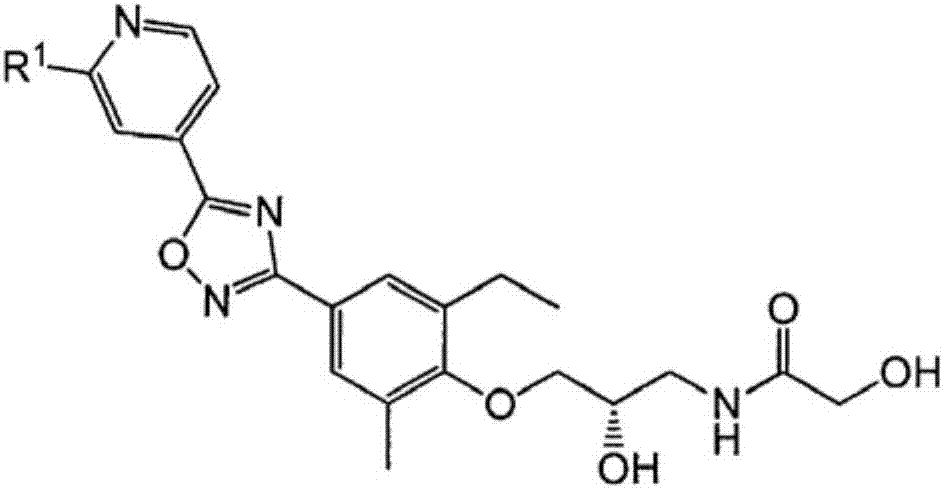
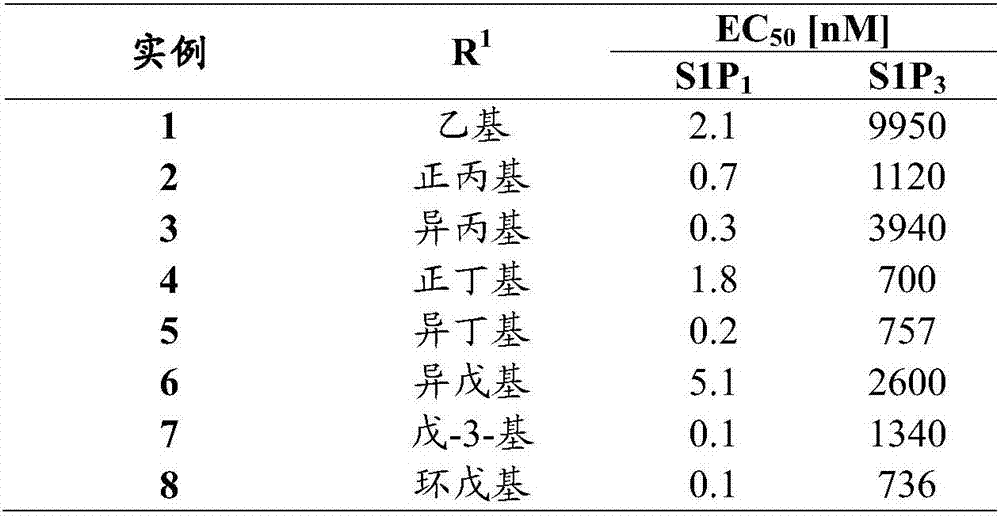
Pyridin-4-yl derivatives
An ethyl and alkyl technology, applied in the field of pyridine-4-yl derivatives, can solve the problems of accelerating organ damage, weakening the immune system's defense against infection and malignant diseases, etc. Effects of end organ damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0144] (S)-N-(3-(2-Ethyl-4-(5-(2-ethylpyridin-4-yl)-1,2,4-oxadiazol-3-yl)-6-methanol Base-phenoxy)-2-hydroxypropyl)-2-glycolamide
[0145]
[0146] EDC HCl (307mg, 1.60mmol) and HOBt (216mg, 1.60mmol) were added to N-((S)-3-[2-ethyl-4-(N-hydroxyformamidoyl)-6-methyl- A solution of phenoxy]-2-hydroxy-propyl)-2-hydroxy-acetamide (290 mg, 1.60 mmol) in DMF (15 mL). The mixture was stirred at room temperature for 20 minutes, then 2-ethyl-isonicotinic acid (347 mg, 1.07 mmol) was added. Stirring was continued for 1 hour at room temperature. The mixture was diluted with ethyl acetate (50 mL) and washed with NaHCO 3It was washed twice with saturated aqueous solution (2×10 mL) and brine (1×10 mL). The organic extract was subjected to MgSO 4 Dry, filter and concentrate. The remaining residue was dissolved in dioxane (8 mL) and the mixture was stirred at 120 °C for 1 hr. The mixture was concentrated and the crude product was purified by preparative HPLC (Waters XBridge 10 μm, ...
example 2
[0148] (S)-N-(3-(2-Ethyl-4-(5-(2-propylpyridin-4-yl)-1,2,4-oxadiazol-3-yl)-6-methanol Base-phenoxy)-2-hydroxypropyl)-2-glycolamide
[0149]
[0150] Preparation is carried out analogously to Example 1. Beige solid; LC-MS: t R =0.68min,[M+1] + =455.22; 1 H NMR (CDCl 3 ):δ8.77(d,J=5.1Hz,1H),7.93(s,1H),7.84-7.89(m,3H),7.20(t br,J=5.8Hz,1H),4.18-4.25(m ,3H),3.90(dd,J 1 =9.6Hz,J 2 =4.7Hz,1H),3.85(dd,J 1 =9.5Hz,J 2 =6.1Hz,1H),3.79(ddd,J 1 =14.1Hz,J 2 =6.5Hz,J 3 =3.0Hz,1H),3.49-3.58(m,1H),2.90-2.96(m,2H),2.75(q,J=7.5Hz,2H),2.39(s,3H),1.81-1.91(m, 2H), 1.32(t, J=7.5Hz, 3H), 1.04(t, J=7.3Hz, 3H); 13 C NMR (CDCl 3 ): δ173.8, 173.7, 168.9, 164.0, 157.4, 150.2, 137.7, 131.7, 131.5, 128.3, 126.6, 122.3, 120.5, 118.7, 74.2, 70.0, 62.1, 42.3, 40.3, 23.0, 22, 31.8, 16.5
example 3
[0152] (S)-N-(3-(2-Ethyl-4-(5-(2-isopropylpyridin-4-yl)-1,2,4-oxadiazol-3-yl)-6- Methylphenoxy)-2-hydroxypropyl)-2-glycolamide
[0153]
[0154] Preparation is carried out analogously to Example 1. Beige solid; LC-MS: t R =0.71min,[M+1] + = 455.20; 1 H NMR (CDCl 3 ):δ8.75(d,J=5.1Hz,1H),7.93(s,1H),7.81-7.86(m,3H),7.37(t,J=5.8Hz,1H),4.16-4.24(m, 3H),3.82-3.92(m,2H),3.78(ddd,J 1 =14.0Hz,J 2 =6.5Hz,J 3 =3.1Hz, 1H), 3.48-3.57(m, 1H), 3.22(septet, J=7.0Hz, 1H), 2.73(q, J=7.5Hz, 2H), 2.37(s, 3H), 1.40( d, J=6.9Hz, 6H), 1.30(t, J=7.5Hz, 3H); 13 C NMR (CDCl 3 ): δ174.0, 173.4, 169.04, 168.99, 157.4, 150.2, 137.7, 131.70, 131.67, 128.4, 126.7, 122.4, 118.9, 118.6, 74.1, 70.1, 62.2, 42.3, 36.5, 22.9, 22.5
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com