Application of two halogen-phenol compounds to effect of promoting angiogenesis
An angiogenesis and compound technology, applied in cardiovascular system diseases, medical preparations containing active ingredients, drug combinations, etc., can solve the problems of low compliance, instability, inconvenient administration, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
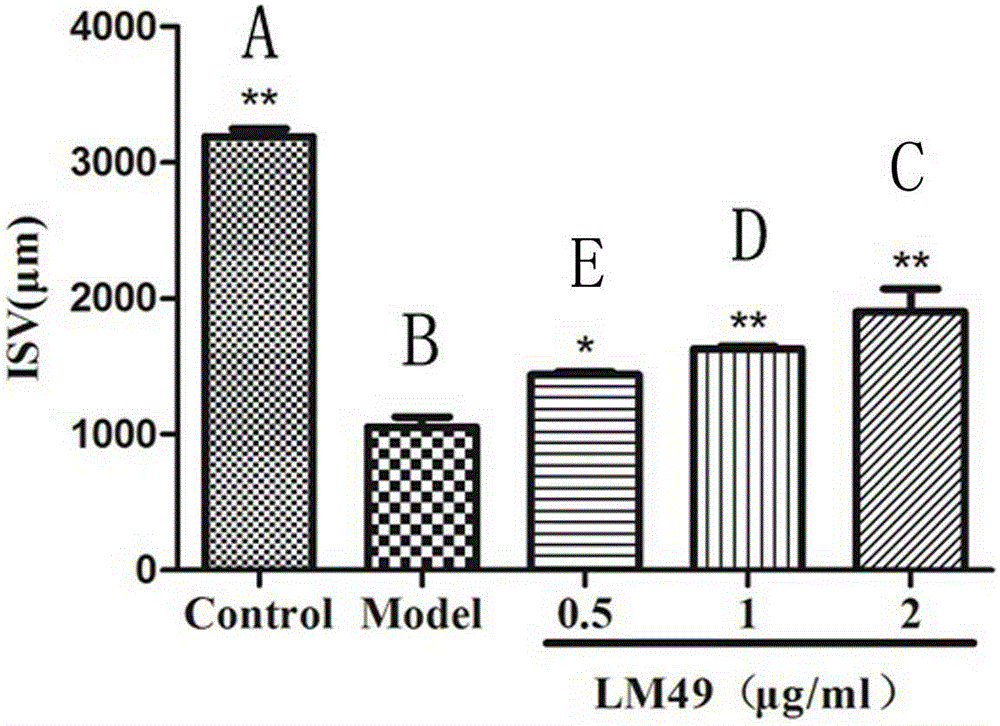
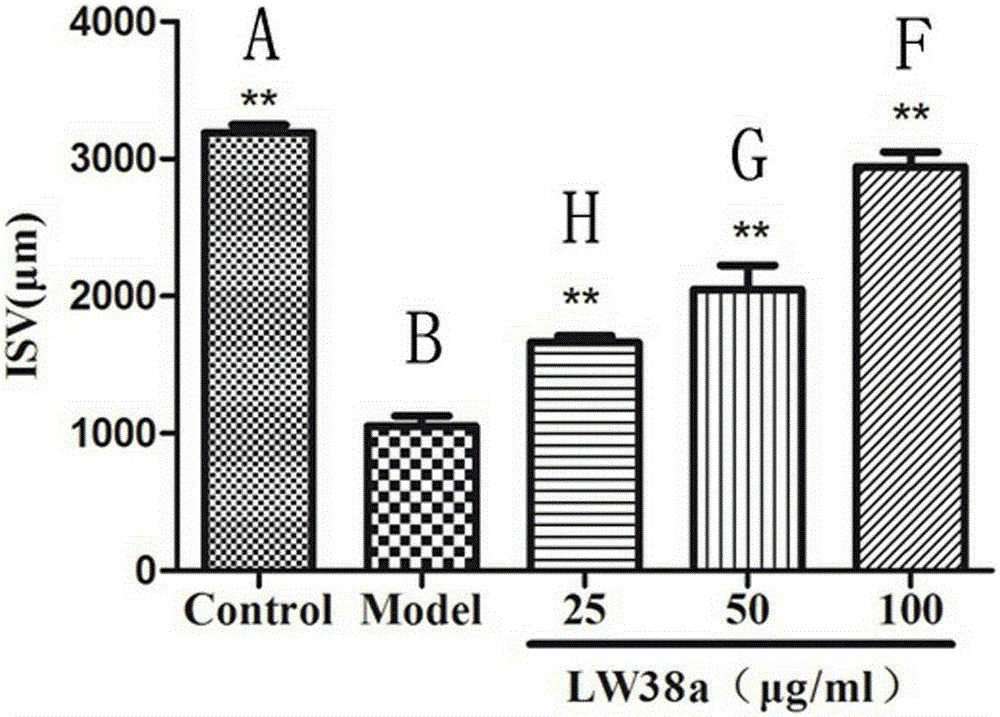
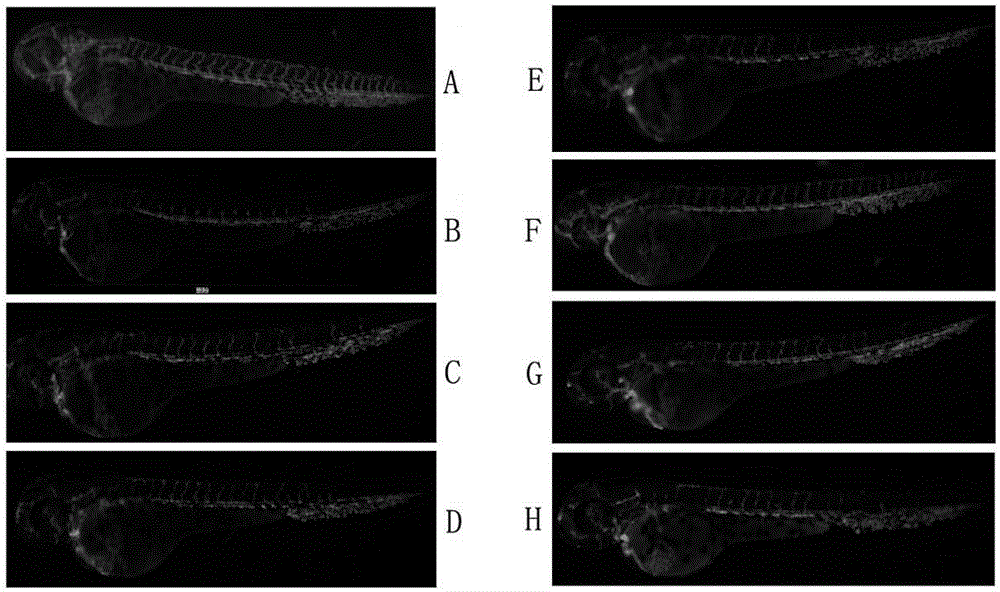
[0014] Example: Halophenol compounds 2,4',5'-trihydroxy-5,2'-dibromobenzophenone and 2',5-dibromo-2-(2-phenylimidazol-1-yl) Effect of methyl-4',5'-dihydroxybenzophenone on PTK787-induced vascular injury in transgenic zebrafish.
[0015] 1. Experimental method
[0016] 1. Preparation of Zebrafish Embryos
[0017] Select a healthy sexually mature female fish and one male fish and put them into the fish tank, and separate the two fish for overnight. The baffle is removed in the morning of the next day, and the male and female fish lay eggs (small grain size, transparent shape). After sterilizing the fertilized eggs, transfer them into the water for zebrafish embryo culture, and culture them under light control at 28°C
[0018] 2. Sample solution preparation
[0019] PTK787, LM49, and LW38a were prepared into 0.5g / L, 20g / L, and 100g / L mother solutions with dimethyl sulfoxide (DMSO), respectively.
[0020] 3. Effect of samples on angiogenesis in transgenic zebrafish
[0021] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com