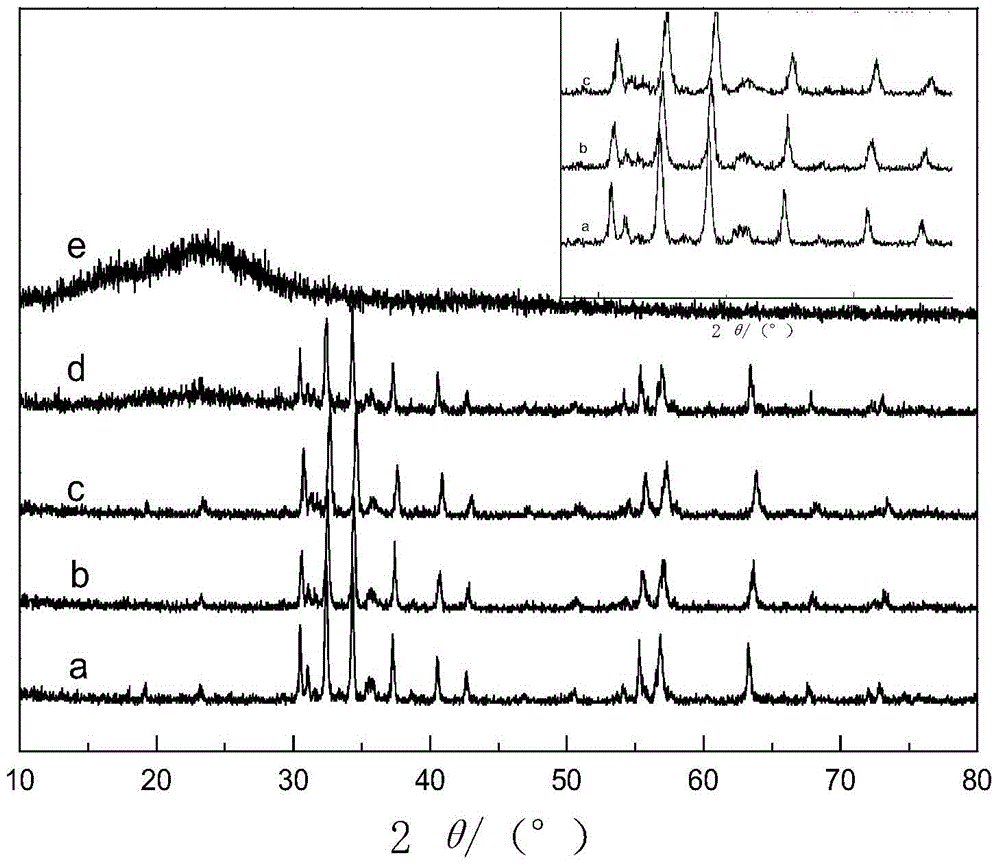
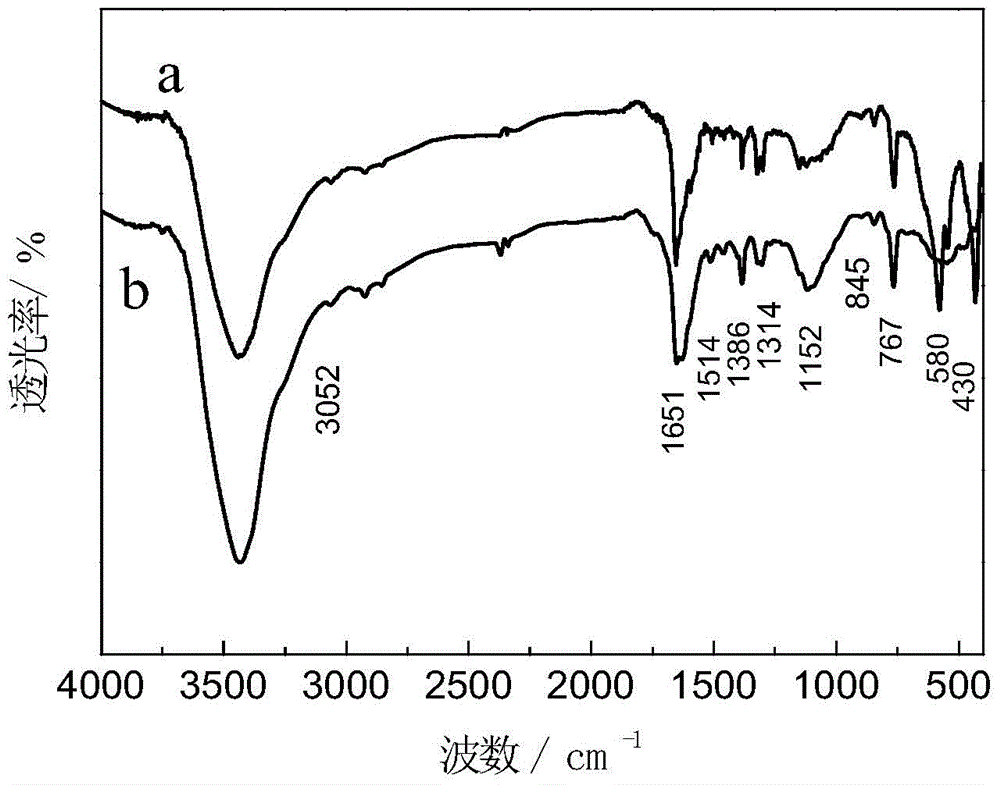
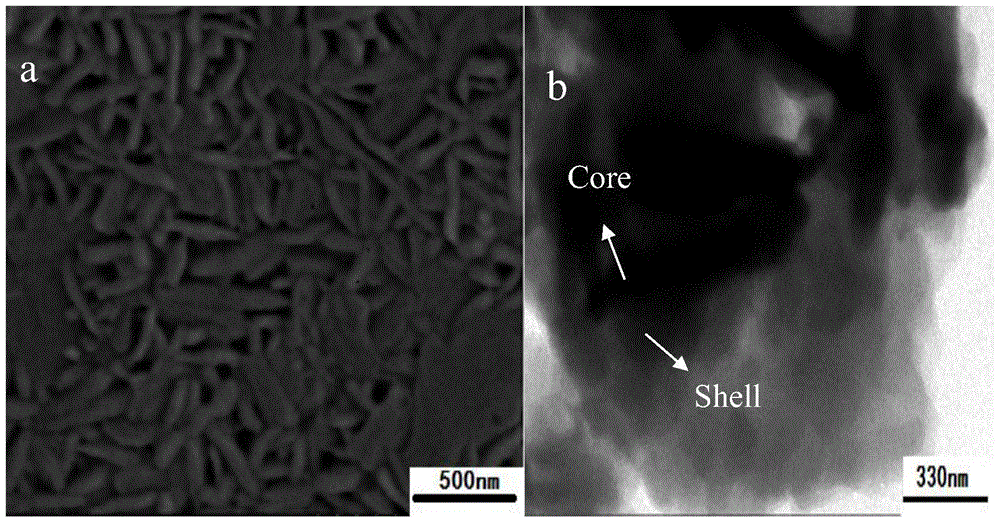
Al-doped Barium Strontium Ferrite-Poly-α-Naphthylamine Composites
A barium strontium iron, aluminum doping technology, applied in iron compounds, inorganic material magnetism, inorganic chemistry, etc., can solve the problems of high density of ferrite materials, insufficient absorption frequency band, low absorption strength, etc., and achieve hard agglomeration content Less, improved absorption efficiency, sufficient effect of complexation reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Aluminum-doped barium strontium ferrite-polyα-naphthylamine (barium strontium ferrite (Ba 0.5 Sr 0.5 Fe 12 o 19 , ie the preparation of x=0)):
[0034](1) Add 4.848g of ferric nitrate nonahydrate, 0.1058g of strontium nitrate, and 0.1307g of barium nitrate into deionized water, stir until dissolved, add 4.098g of citric acid monohydrate, mix well, add concentrated ammonia dropwise to adjust PH=7. After ultrasonication for 1 hour, heat and stir in a water bath at about 80°C until a viscous wet gel is formed. The wet gel was vacuum-dried in a drying oven at 120°C until the water evaporated completely to form a xerogel. After grinding, it was kept at 400° C. for 2 hours, then raised to 800° C. at a heating rate of 10° C. / min, and kept at 4 hours to obtain brown barium strontium ferrite.
[0035] (2) Weigh 0.5g of barium strontium ferrite and add it to 50mL of 0.15mol / L hydrochloric acid solution, ultrasonically oscillate for 30 minutes to disperse them evenly, then sl...
Embodiment 2
[0037] Aluminum-doped barium strontium ferrite-polyα-naphthylamine composite material (aluminum-doped barium strontium ferrite Ba 0.5 Sr 0.5 Fe 11.5 Al 0.5 o 19 , i.e. the preparation of x=0.5):
[0038] (1) accurately take by weighing 4.646g ferric nitrate nonahydrate, 0.1307g barium nitrate, 0.1058g strontium nitrate and 0.1875g aluminum nitrate nonahydrate, all the other are with the step (1) in the embodiment 1, promptly get the Ba of x=0.5 0.5 Sr 0.5 Fe 11.5 Al 0.5 o 19 Ferrite;
[0039] (2) Weigh 0.45gBa 0.5 Sr 0.5 Fe 11.5 Al 0.5 o 19 Aluminum-doped barium strontium ferrite, 45mL0.15mol / L hydrochloric acid solution, ultrasonic vibration for 30 minutes to make them uniformly dispersed, then slowly add the analytically pure 0.9g α-naphthylamine monomer into the doped barium strontium ferrite In the hydrochloric acid suspension of the oxygen body, continue to sonicate for about 30 minutes. The mixed solution was transferred to an ice-water bath, and 1.4344 g ...
Embodiment 3
[0041] Aluminum-doped barium strontium ferrite-polyα-naphthylamine composite material (aluminum-doped barium strontium ferrite Ba 0.5 Sr 0.5 Fe 12-x Al x o 19 , ie the preparation of x=1):
[0042] (1) accurately take by weighing 4.444g ferric nitrate nonahydrate, 0.1307g barium nitrate, 0.1058g strontium nitrate and 0.3751g aluminum nitrate nonahydrate (molar content is 0.005), all the other are with the step (1) in the embodiment 1, to obtain final product Ba for x=1 0.5 Sr 0.5 Fe 12- x Al x o 19 Ferrite;
[0043] (2) Weigh 0.4gBa 0.5 Sr 0.5 Fe 11.5 Al 0.5 o 19 Aluminum-doped barium strontium ferrite, 40mL0.15mol / L hydrochloric acid solution, ultrasonic vibration for 30 minutes to make them uniformly dispersed, and then slowly add 0.8g of analytically pure α-naphthylamine monomer into the doped barium strontium ferrite In the hydrochloric acid suspension of the oxygen body, continue to sonicate for about 30 minutes. The mixed solution was transferred to an i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption bandwidth | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com