Hepatic abscess-causing Klebsiella pneumoniae specific gene sequence asnc and its application
A technology for Klebsiella pneumoniae and Klebsiella liver, which is applied to the specific sequence asnC of Klebsiella pneumoniae gene and its application field, which can solve the easy missed diagnosis and delay the diagnosis of Klebsiella pneumoniae liver abscess , Low culture positive rate and other issues, to achieve high specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The screening process of specific gene sequences combines the next-generation sequencing technology (Next-Generation Sequencing, NGS), comparative genomics (Comparative Genomics) and bioinformatics (Bioinformatics) methods, and uses scientific statistical methods to analyze sequences in multiple The specificity of the distribution in the Klebsiella pneumoniae genome was tested, and PCR validation was performed in the isolated Klebsiella pneumoniae and non-hepatoabscess Klebsiella pneumoniae to determine the sequence in Klebsiella pneumoniae. Specificity in Lebsiella. The specific implementation process is as follows:
[0035] Pathogen isolation and identification:
[0036] 1. Enrollment of patients with suspected Klebsiella pneumoniae liver abscess. Patients with clinically suspected Klebsiella pneumoniae liver abscess were screened according to the following criteria:
[0037] (1) Clinical manifestations of the patient: fever, chills and abdominal pain;
[0038] (2...
Embodiment 2
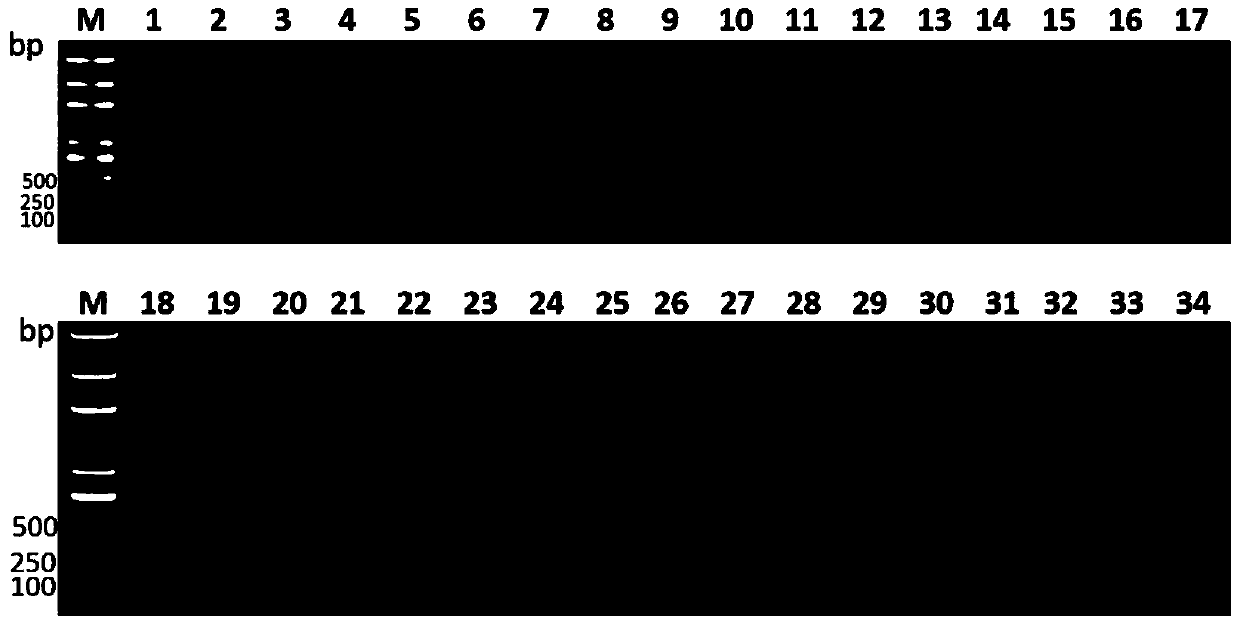
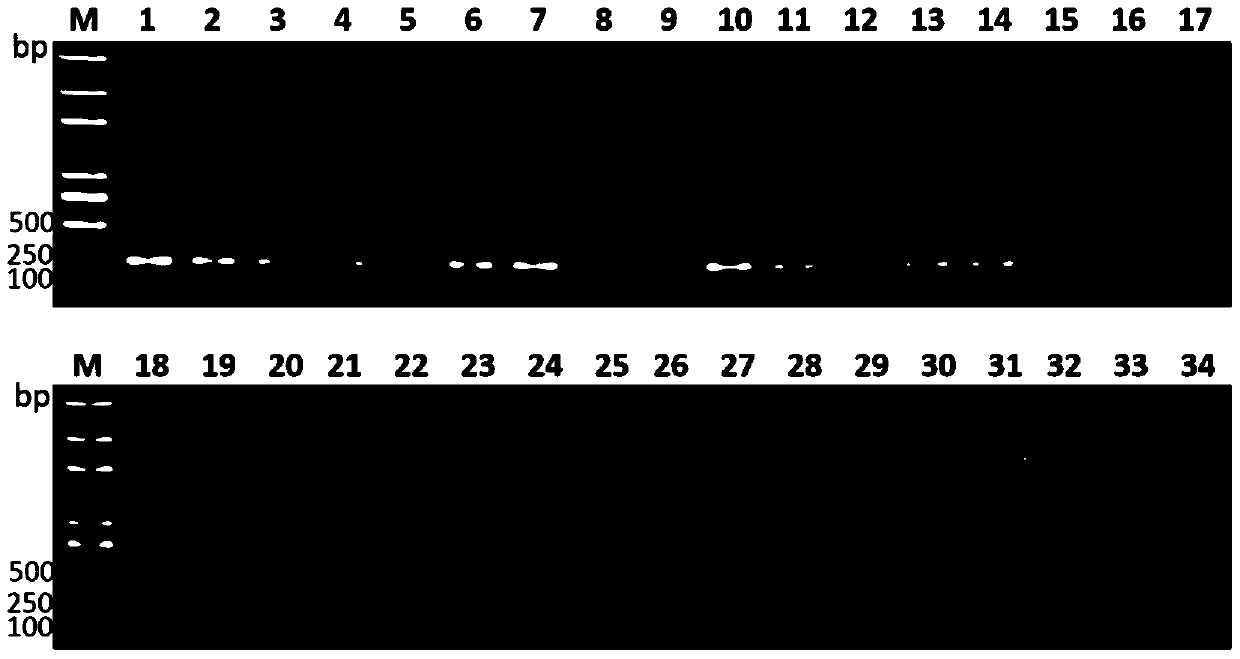
[0061]Gene-specific verification:
[0062] The specific steps for the verification of three hepatic abscess-causing Klebsiella pneumoniae-specific genes in clinically isolated strains are:
[0063] 1. Select clinically isolated hepatic abscess and non-hepatic abscess Klebsiella pneumoniae bacterial strains for detection of sdm (shown in SEQ ID NO.1), lak (shown in SEQ ID NO.3) and asnC (shown in SEQ ID NO.3) .2) specificity in hepatoabscess-causing Klebsiella pneumoniae. 17 strains of Klebsiella pneumoniae isolated from the abscess of patients with liver abscess were selected as the detection group (hepatic abscess group), and 17 strains of Klebsiella pneumoniae isolated from non-Klebsiella pneumoniae patients with liver abscess were selected as negative controls group (non-hepatic abscess-induced group). At the same time, other common pathogenic bacteria (other pathogenic bacteria groups) isolated clinically were also selected to further verify the specificity of these fact...
Embodiment 3
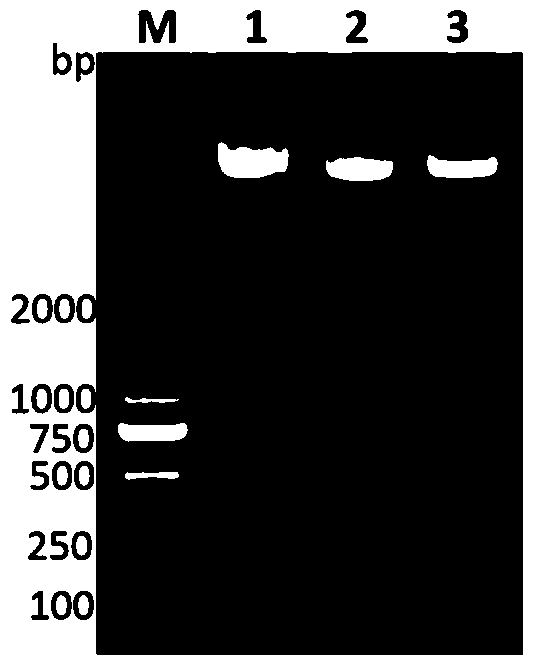
[0086] Application of sdm, lak and asnC sequences or primers designed based on their sequences in the preparation of Klebsiella pneumoniae liver abscess diagnostic kit:
[0087] The design idea of this experiment is as follows: the clinically obtained blood culture samples are divided into a blood culture positive group and a healthy person negative group, where the blood culture positive group refers to all samples with bacteria cultured in the blood, not limited to the blood cultured with pneumonia. Lebsiella, including cultures of other pathogenic bacteria. Use PCR to detect the presence of specific factors (i.e. sdm, lak and asnC) in positive blood culture samples and negative blood culture samples, and finally analyze the corresponding analysis of the PCR results and the patient's final clinical diagnosis, so as to judge whether the method provided by the present invention is suitable for Detection rate of Klebsiella pneumoniae liver abscess.
[0088] 1. Collection of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com