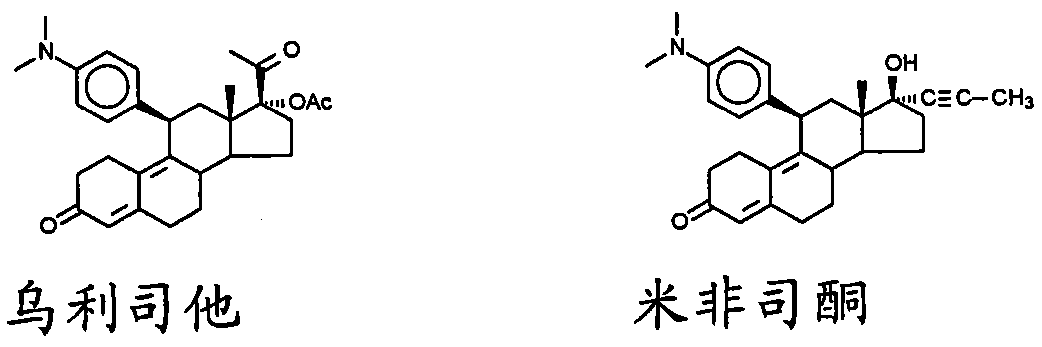
imidazolyl progesterone antagonist
A technology of imidazolyl and alkyl, which is applied in the field of preparation of new compounds and can solve problems such as omission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0219] Nuclear receptor analysis
[0220] Using GeneBLAzer TM Invitrogen’s β-lactamase reporter technology The Cell Based Nuclear Receptor Assay service performs the determination of the agonist / antagonist properties of test compounds. Essentially this assay uses a β-lactamase cDNA under the transcriptional control of an upstream activating sequence (UAS). UAS is activated by the GAL4 transcription factor DNA-binding domain (DBD), which is expressed as a fusion protein containing the ligand-binding domain (LBD) of the target receptor. Upon ligand binding, GAL4(DBD)-NR(LDB) binds to UAS, thereby controlling the transcription of β-lactamase. β-lactamases cleave specially engineered fluorogenic substrates, resulting in a change in the measured fluorescence wavelength.
[0221] The general protocol for progesterone antagonist screening initiated by the control agonist R5020 is as follows:
[0222] Progesterone receptor-LBD-UAS-bla HEK 293T cells were thawed and prepared as...
Embodiment 2
[0228] Pregnant guinea pig model as described by Walter Elger et al., J. Steroid Biochem, Vol. 25, No. 5B, pp. 835-845, 1986:
[0229] Adult female guinea pigs weighing approximately 500 g were housed and tested for cycle status by daily inspection of the vaginal opening.
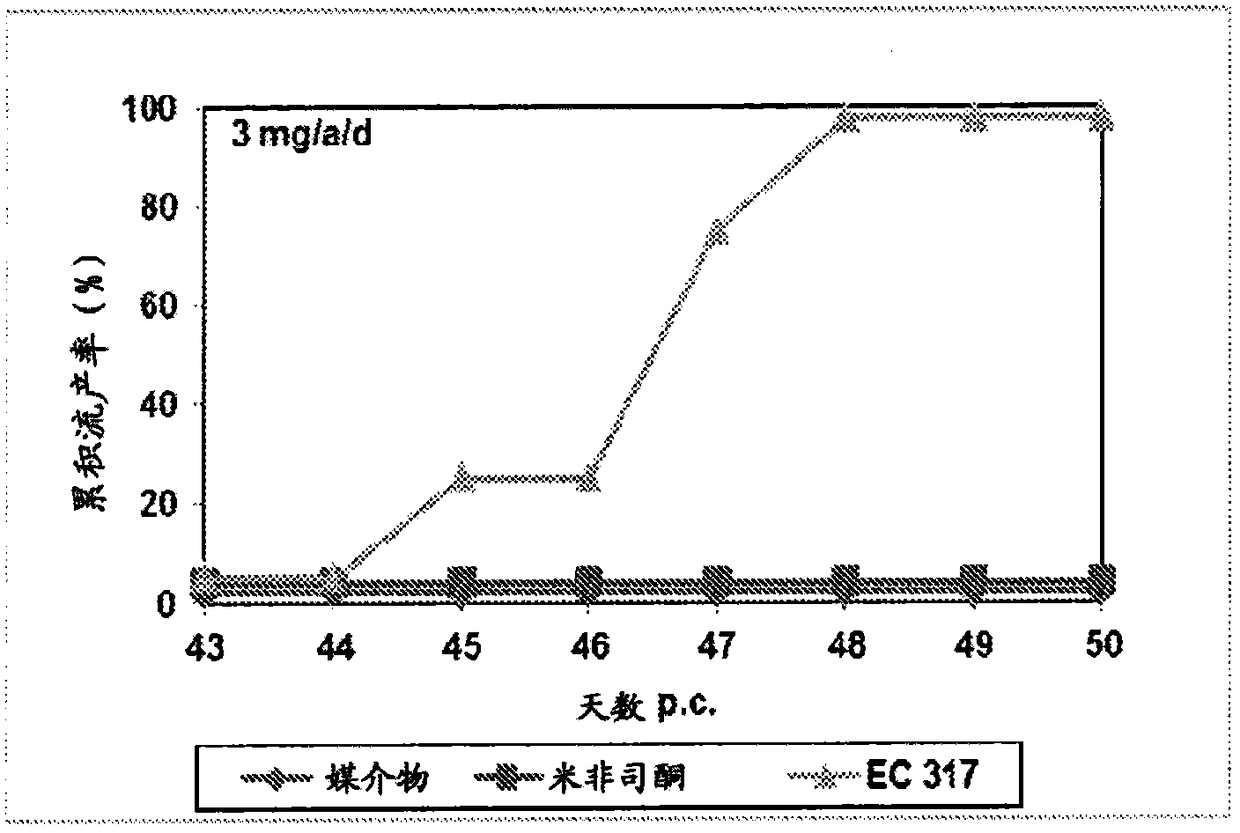
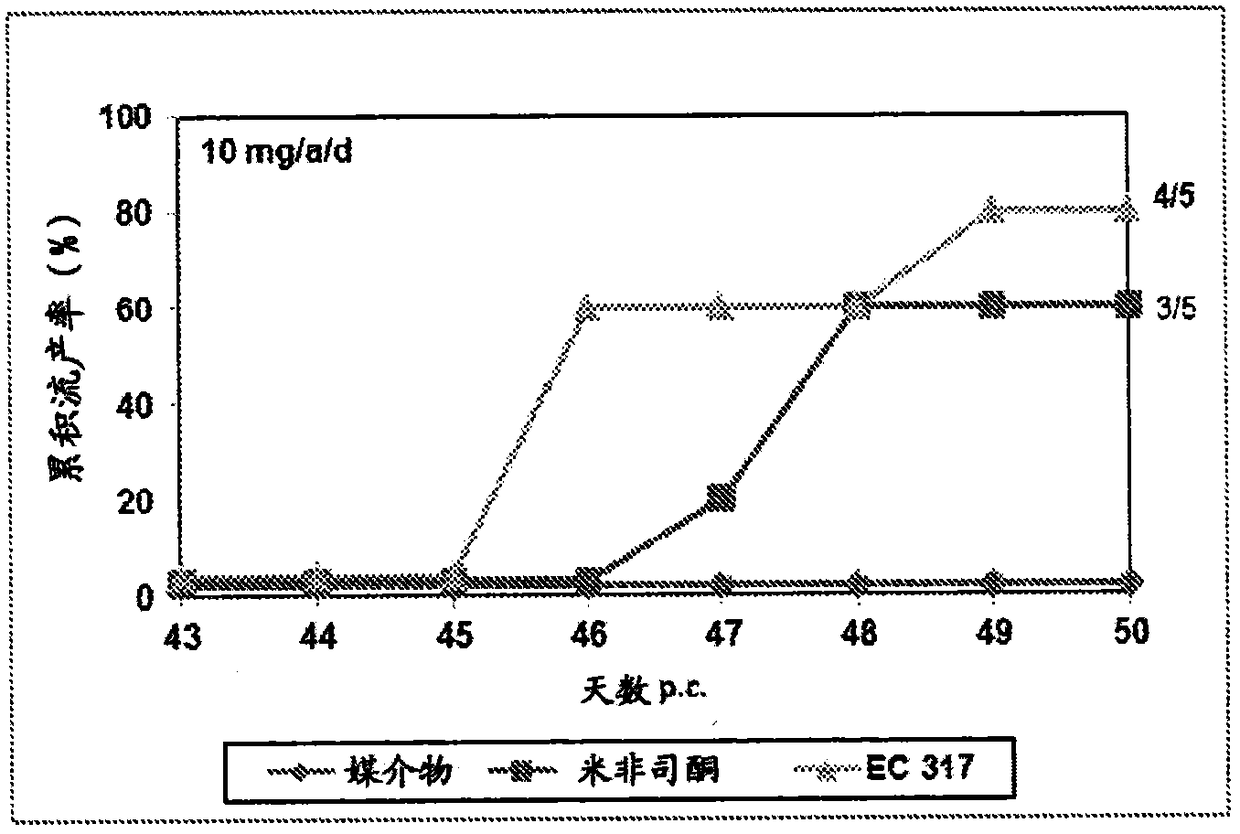
[0230] In the second period, three females were co-housed with one male on day 15 following day 1 of testing the vaginal opening. Day 16 of the cycle was considered pregnancy day 1. Pregnant animals were randomized into different treatment groups and treated subcutaneously on days 43 and 44 of gestation with the test substances dissolved in 0,2 ml benzyl benzoate / castor oil. Animals were examined for vaginal bleeding and the number and timing of abortions. figure 1 shows a test result of 3mg / a / d, while figure 2 Test results for 10 mg / a / d are shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com