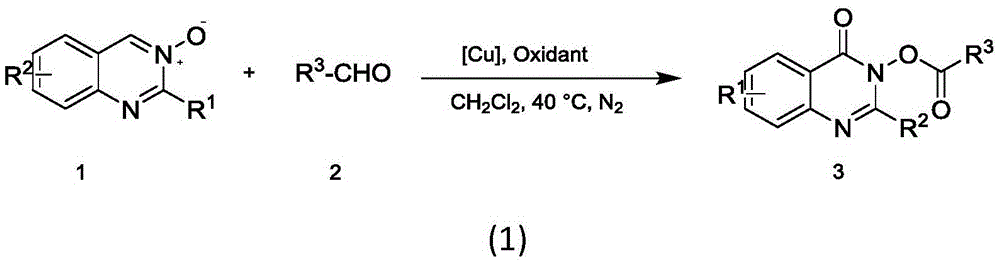
Method for efficiently preparing quinazolinone compound
A technology for quinazolinones and compounds, which is applied in the field of efficient preparation of quinazolinone compounds, can solve the problems of inability to achieve substitution, high reaction temperature, limited product types of quinazolinone compounds, etc., and achieves less limitation of substrates , the reaction temperature is low, the effect of realizing the application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0020]
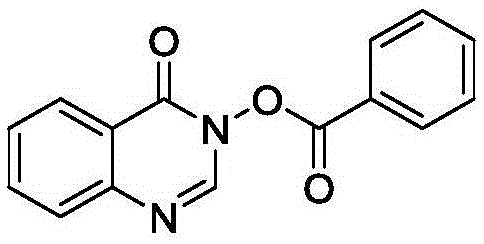
[0021] Add 0.2mmol 3-oxoquinazoline and 0.02mmol copper acetate to a clean 25mL reaction tube, and then add 0.6mmol benzaldehyde, 0.6mmol tert-butyl hydroperoxide (5.5 M in decane) and 2 mL of dichloromethane were reacted at 40 ° C for 4 hours, and detected by spotting on a silica gel plate; after the reaction, the reaction solution was extracted 3-5 times with ethyl acetate, and the organic phases were combined and dried with anhydrous sodium sulfate , the organic layer was concentrated and subjected to column chromatography to obtain pure benzoic acid-4-oxo-4-hydroquinazolin-3-ester as a pale yellow solid with a yield of 84%.
[0022] 1 HNMR (400MHz, CDCl 3 )δ8.34(d, J=8.2Hz, 1H), 8.21(d, J=7.9Hz, 2H), 8.15(s, 1H), 7.85–7.78(m, 2H), 7.74–7.67(m, 1H ),7.58–7.51(m,3H).
[0023] 13 CNMR (100MHz, CDCl 3 )δ163.0, 156.0, 147.1, 143.3, 135.1, 134.7, 130.6, 129.0, 128.1, 127.7, 127.1, 125.3, 123.6.
[0024] HRMS(ESI)calcdforC 15 h 10 N 2 o 3 [M+Na] + :289.0589...
example 2
[0028]
[0029] Add 0.2mmol 2-methyl-3-oxoquinazoline and 0.02mmol copper acetate to a clean 25mL reaction tube, and then add 0.6mmol benzaldehyde and 0.6mmol tert-butyl Hydrogen peroxide (5.5M in decane) and 2 mL of dichloromethane were reacted at 40°C for 4 hours, and detected by spotting on a silica gel plate; after the reaction, the reaction solution was extracted 3-5 times with ethyl acetate, and the organic phases were combined and used After drying over anhydrous sodium sulfate, the organic layer was concentrated and subjected to column chromatography to obtain pure benzoic acid-2-methyl-4-oxo-4-hydroquinazolin-3-ester as a pale yellow solid with a yield of 88%.
[0030] 1 HNMR (400MHz, CDCl 3 )δ8.27(d, J=8.0Hz, 1H), 8.23(d, J=8.1Hz, 2H), 7.82–7.76(m, 1H), 7.76–7.68(m, 2H), 7.60–7.53(m ,2H),7.53–7.46(m,1H),2.55(s,3H).
[0031] 13 CNMR (100MHz, CDCl 3 )δ162.8, 156.5, 152.3, 146.5, 135.1, 134.8, 130.6, 129.0, 127.2, 127.1, 126.8, 125.5, 122.3, 20.1.
[0032] HRMS...
example 3
[0034]
[0035] Add 0.2mmol 2-methyl-3-oxoquinazoline and 0.02mmol copper acetate into a clean 25mL reaction tube, and after pumping 3 times under a nitrogen atmosphere, add 0.6mmol p-tolualdehyde, 0.6mmol Tert-butyl hydroperoxide (5.5M in decane) and 2 mL of dichloromethane were reacted at 40°C for 4 hours, and detected by spotting on a silica gel plate; after the reaction, the reaction solution was extracted 3-5 times with ethyl acetate, and combined The organic phase was dried over anhydrous sodium sulfate, the organic layer was concentrated and subjected to column chromatography to obtain pure 4-methylbenzoic acid-2-methyl-4-oxo-4-hydroquinazolin-3-ester as a light yellow solid , yield 93%.
[0036] 1 HNMR (400MHz, CDCl 3 )δ8.27(d,J=8.0Hz,1H),8.12(d,J=8.2Hz,2H),7.81–7.75(m,1H),7.71(d,J=8.2Hz,1H,),7.51 –7.45(m,1H),7.36(d,J=8.1Hz,2H),2.54(s,3H),2.47(s,3H).
[0037] 13CNMR (100MHz, CDCl 3 )δ162.8, 156.6, 152.4, 146.6, 146.3, 134.7, 130.6, 129.8, 127.2, 127.0, 126.8, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com