Barbiturate compound, preparing method and application thereof
A technology of barbituric acid and compounds, applied in the field of medicinal chemistry, can solve the problems that cannot be ruled out, the selectivity is not high, the inhibitory effect is not strong, etc., and the effect of good inhibitory activity can be achieved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
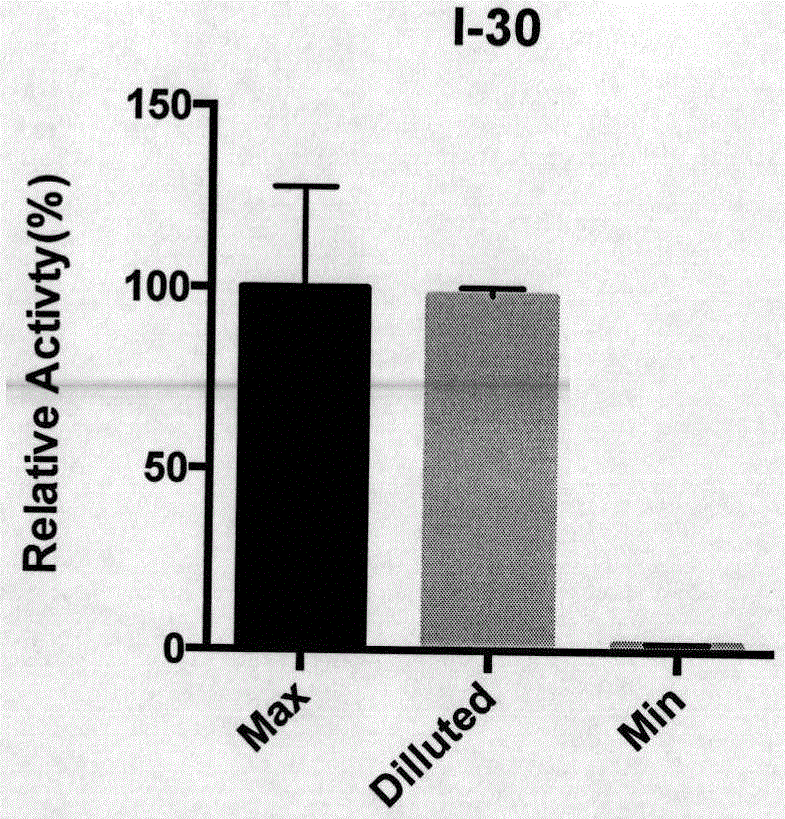
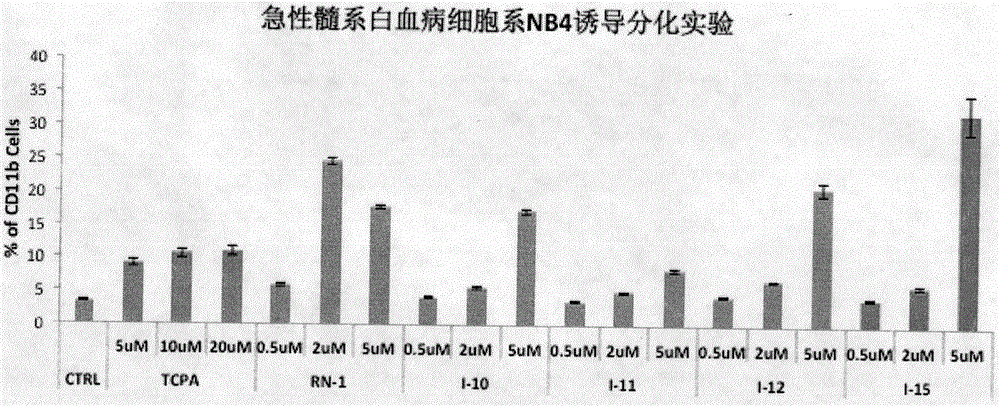
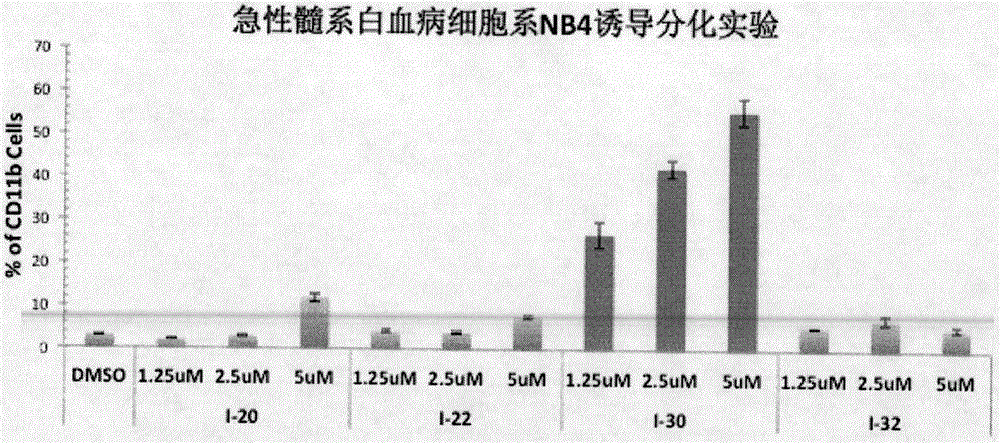
Image
Examples
Embodiment 1
[0036] (E / Z)-5-((1H-indol-2-yl)methylene)-1-(4-bromophenyl)pyrimidine-2,4,6(1H,3H,5H)-trione Preparation of (I-1)
[0037] (1) Indole-2-carbaldehyde
[0038]
[0039]Add 805.8 mg (5 mmol) of indole-2-carboxylic acid to a 100 mL eggplant-shaped bottle, and add THF 30 mL. Slowly add LiAlH while stirring in an ice bath 4 379.5 mg (10 mmol). Stir at room temperature for 14 h until the end of the reaction detected by TLC. Add saturated NH 4 Cl solution, and the organic layer was extracted three times with ethyl acetate. Wash with brine, combine the organic layers, and dry over anhydrous sodium sulfate. The solvent was spin-dried under reduced pressure to obtain 441.5 mg of yellow crude indole-2-methanol, with a yield of 60%. The next reaction was carried out directly without purification.
[0040] Crude indole-2-methanol 441.5mg (3mmol) was dissolved in 20mLCH 2 Cl 2 , adding activated MnO 2 1.74g (20mmol), stirred at room temperature for 18h until the end of the reac...
Embodiment 2
[0051] (E / Z)-5-((1H-indol-2-yl)methylene)-1-(4-chlorophenyl)pyrimidine-2,4,6(1H,3H,5H)-trione Preparation of (I-2)
[0052] (1) 1-(4-Chlorophenyl)urea
[0053]
[0054] Referring to Example 1, 1-(4-chlorophenyl)urea was synthesized using p-chloroaniline as a raw material with a yield of 72.3%. 1 HNMR (300MHz, DMSO-d 6 )δ8.78(s, 1H, -N H -), 7.71 (d, J=8.4Hz, 2H, Ar H ), 7.38 (d, J=8.4Hz, 2H, Ar H ), 5.93(s, 2H, -N H 2 ).
[0055] (3) 1-(4-Chlorophenyl)pyrimidine-2,4,6(1H,3H,5H)-trione
[0056]
[0057] Reference Example 1, using 1-(4-chlorophenyl)urea as raw material to synthesize 1-(4-chlorophenyl)pyrimidine-2,4,6(1H,3H,5H)-trione with a yield of 52.0% . 1 HNMR (300MHz, DMSO-d 6 )δ11.51(s, 1H, -N H -), 7.54 (d, J=8.4Hz, 2H, Ar H ), 7.09 (d, J=8.4Hz, 2H, Ar H ), 3.72(s, 2H, -C H 2 -).
[0058] (4) (E / Z)-5-((1H-indol-2-yl)methylene)-1-(4-chlorophenyl)pyrimidine-2,4,6(1H,3H,5H) - Triketone (I-2)
[0059]
[0060] Referring to Example 1, using 1-(4-chl...
Embodiment 3
[0062] (E / Z)-5-((1Hindol-2-yl)methylene)-1-(3-nitrophenyl)pyrimidine-2,4,6(1H,3H,5H)-trione Preparation of (I-3)
[0063] (1) 1-(3-nitrophenyl)urea
[0064]
[0065] Referring to Example 1, 1-(3-nitrophenyl)urea was synthesized from m-nitroaniline with a yield of 63.0%. 1 HNMR (300MHz, DMSO-d 6 )δ8.91(s, 1H, -N H -), 8.35(s, 1H, Ar H ), 7.95 (m, 2H, Ar H ), 7.59(t, J=8.4Hz, 1H, Ar H ), 5.90(s, 2H, -N H 2 ).
[0066] (3) 1-(3-nitrophenyl)pyrimidine-2,4,6(1H,3H,5H)-trione
[0067]
[0068] Reference Example 1, using 1-(3-nitrophenyl)urea as raw material to synthesize 1-(3-nitrophenyl)pyrimidine-2,4,6(1H,3H,5H)-trione, yield 50.2%. 1 HNMR (300MHz, DMSO-d 6 )δ11.61(s, 1H, -N H -), 8.29 (d, J=6.8Hz, 1H, Ar H ), 8.2 (s, 1H, Ar H ), 7.82-7.75 (m, 2H, Ar H ), 3.76(s, 2H, -C H 2 -).
[0069] (4) (E / Z)-5-((1H-indol-2-yl)methylene)-1-(3-nitrophenyl)pyrimidine-2,4,6(1H,3H,5H )-triketone (I-3)
[0070]
[0071] Referring to Example 1, 1-(3-nitrophenyl)pyrimidine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com