A kind of synthetic method of 2-amino-4-(ethylsulfonyl)phenol
A technology of ethanesulfonyl and synthesis method, which is applied in the field of synthesis of 2-amino-4-phenol, can solve the problems of harsh reaction process, poor product quality, high production cost, etc., achieve activity enhancement, reduce production cost and improve product quality Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
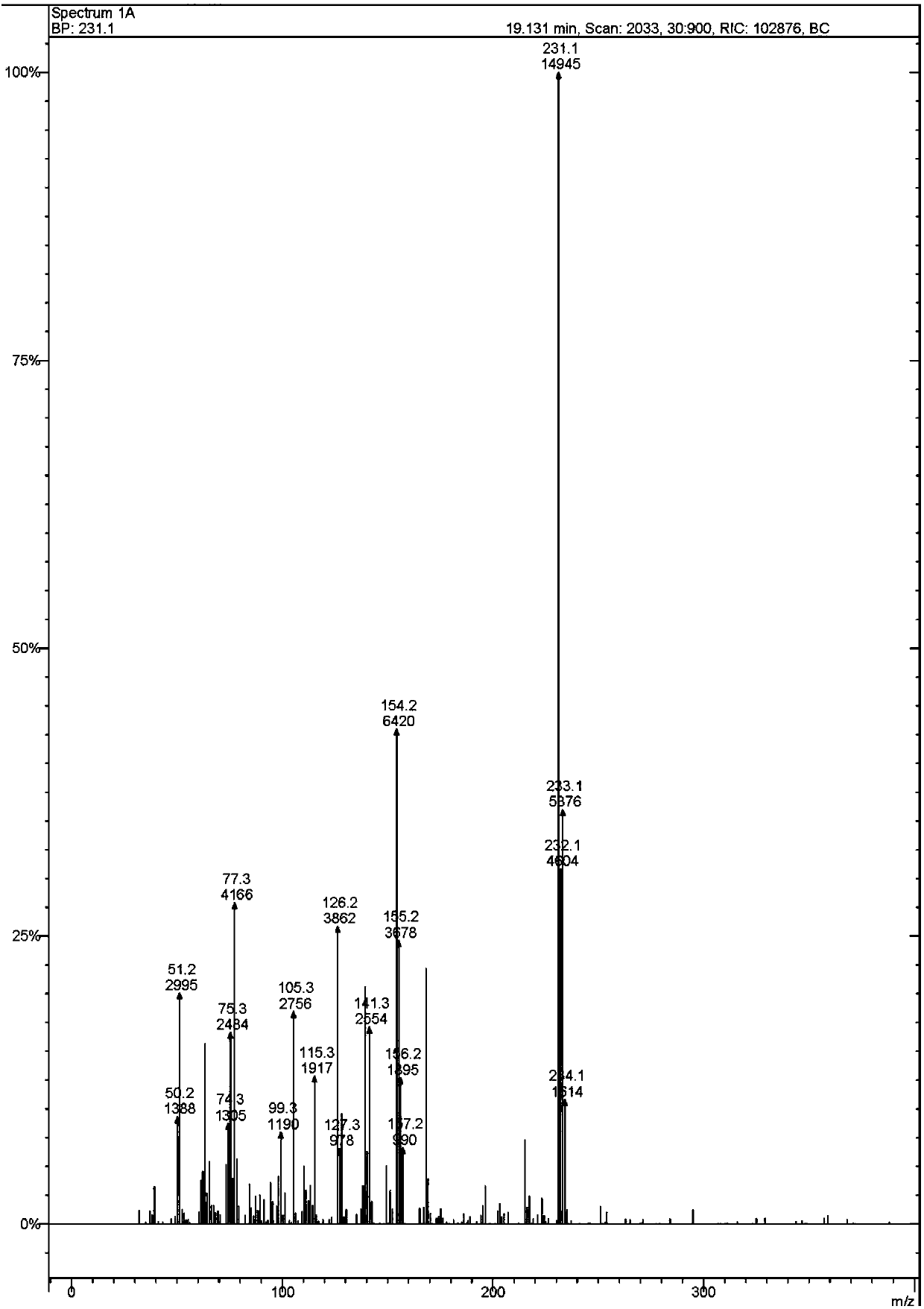
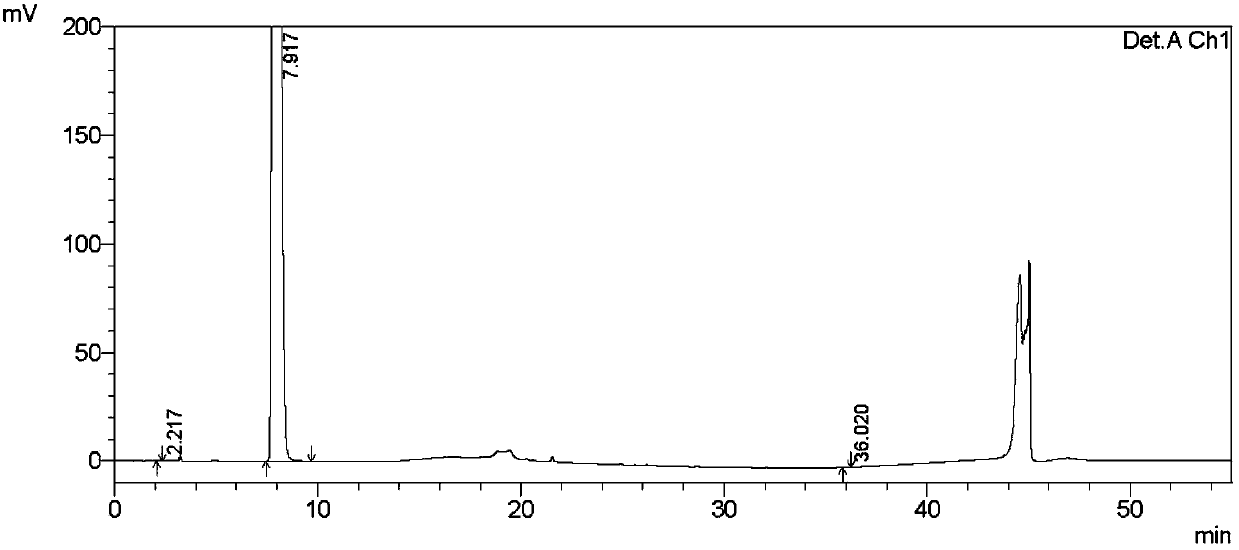
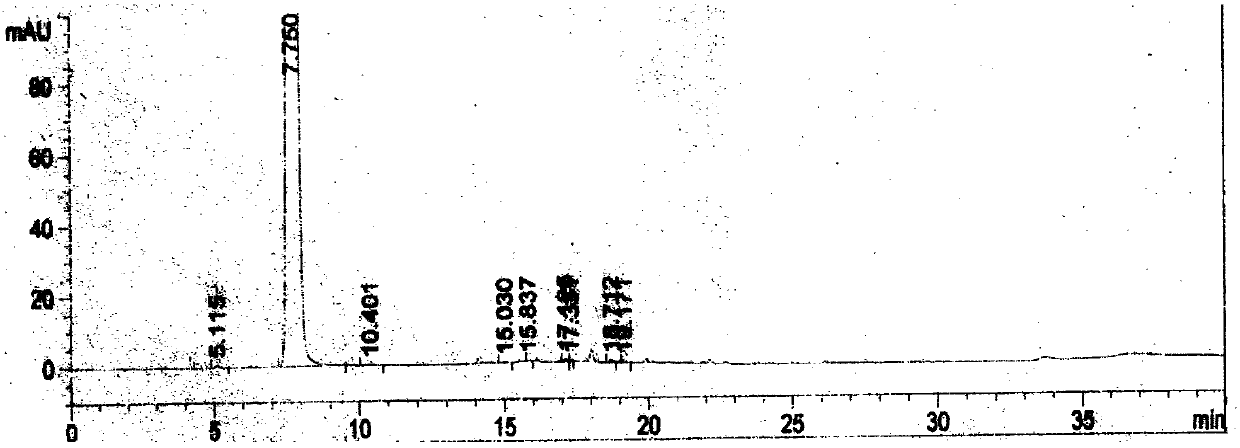
Image
Examples
preparation example Construction
[0036] The invention provides a kind of synthetic method of 2-amino-4-(ethylsulfonyl)phenol, comprising the following steps:
[0037] a) After reacting 4-ethanesulfonyl-2-nitro-chlorobenzene with a base in a solvent, 4-(ethylsulfonyl)-2-nitro-phenol is obtained;
[0038] b) Reducing the 4-(ethylsulfonyl)-2-nitro-phenol obtained in the above steps to obtain 2-amino-4-(ethylsulfonyl)phenol.
[0039] In the present invention, 4-(ethylsulfonyl)-2-nitro-chlorobenzene is reacted with a base in a solvent to obtain 4-(ethylsulfonyl)-2-nitro-phenol.
[0040] The present invention is not particularly limited to the 4-ethanesulfonyl-2-nitro-chlorobenzene, 4-ethanesulfonyl-2-nitro-chlorobenzene well-known to those skilled in the art can be used, and the 4-nitro-chlorobenzene described in the present invention -Ethyl-2-nitro-chlorobenzene preferably has the structure of formula IV,
[0041]
[0042] The present invention is not particularly limited to the 4-(ethanesulfonyl)-2-nitro-ph...
Embodiment 1
[0081] 4-Ethylsulfonyl-chlorobenzene
[0082] Add 100g of 4-chlorophenethylsulfide, 1.0g of sodium tungstate dihydrate and 300mL of ethyl acetate in sequence to a 2L glass reaction bottle; at 40°C, start to add 35% H 2 o 2 190g, control the temperature at 40-50°C, keep warm for half an hour after dripping, then raise the temperature to 55°C, and react for 3 hours; TLC monitors that the reaction is complete, cool down to 20-30°C, add saturated Na 2 SO 3 solution until the potassium iodide test paper does not change color. Stir evenly, stand to separate the organic layer, extract the aqueous layer with 400ml ethyl acetate, combine the organic layers, wash with 300ml brine, dry over anhydrous sodium sulfate, and concentrate to obtain 125g colorless oily 2-nitro-4-ethanesulfonate The crude product of acyl chloride benzene was directly used in the next reaction.
[0083] 4-Ethylsulfonyl-2-nitro-chlorobenzene
[0084] To a 1L glass reaction bottle, add 100g of crude 2-nitro-4-...
Embodiment 2
[0100] 4-Ethylsulfonyl-chlorobenzene
[0101] Into a 250ml glass reaction bottle, sequentially add 20g 4-chlorophenethyl sulfide, 0.2g sodium tungstate dihydrate and 60mL acetic acid; at 40°C, start to drop 40g of 35% H 2 o 2 , control the temperature at 40-50°C, after dropping, keep warm for half an hour, then raise the temperature to 55°C, and react for 3 hours; TLC monitors that the reaction is complete, cool down to 20-30°C, add saturated Na 2 SO 3 solution until the potassium iodide test paper does not change color. Extracted 3 times with 100ml ethyl acetate, combined the organic layers, washed 3 times with 100ml brine, dried over anhydrous sodium sulfate, concentrated to obtain about 25g colorless oily 2-nitro-4-ethanesulfonylchlorobenzene crude product, directly used react in the next step.
[0102] 4-Ethylsulfonyl-2-nitro-chlorobenzene
[0103] Add 25g of crude 4-ethanesulfonyl-chlorobenzene and 75ml of glacial acetic acid to a 250ml glass reaction bottle, cool do...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com